Abstract
A 20-d experiment was conducted to test the hypothesis that phytase increases nutrient digestibility, bone ash, and growth performance of pigs fed diets containing 0.23%, 0.29%, or 0.35% phytate-bound P. Within each level of phytate, five diets were formulated to contain 0, 500, 1,000, 2,000, or 4,000 phytase units (FTU)/kg of a novel phytase (PhyG). Three reference diets were formulated by adding a commercial Buttiauxella phytase (PhyB) at 1,000 FTU/kg to diets containing 0.23%, 0.29%, or 0.35% phytate-bound P. A randomized complete block design with 144 individually housed pigs (12.70 ± 4.01 kg), 18 diets, and 8 replicate pigs per diet was used. Pigs were adapted to diets for 15 d followed by 4 d of fecal collection. Femurs were collected on the last day of the experiment. Results indicated that diets containing 0.35% phytate-bound P had reduced (P < 0.01) digestibility of Ca, P, Mg, and K compared with diets containing less phytate-bound P. Due to increased concentration of total P in diets with high phytate, apparent total tract digestible P and bone ash were increased by PhyG to a greater extent in diets with 0.29% or 0.35% phytate-bound P than in diets with 0.23% phytate-bound P (interaction, P < 0.05). At 1,000 FTU/kg, PhyG increased P digestibility and bone P more (P < 0.05) than PhyB. The PhyG increased (P < 0.01) pig growth performance, and pigs fed diets containing 0.35% or 0.29% phytate-bound P performed better (P < 0.01) than pigs fed the 0.23% phytate-bound P diets. In conclusion, the novel phytase (i.e., PhyG) is effective in increasing bone ash, mineral digestibility, and growth performance of pigs regardless of dietary phytate level.
Keywords: bone ash, digestibility, growth performance, phytase, phytate, pigs
Introduction
Most cereal grains and plant protein ingredients included in swine diets contain phytate, which consists of six phosphate molecules bound to a myo-inositol ring (Zimmermann et al., 2002; Raboy, 2003). Pigs do not synthesize adequate amounts of endogenous phytase to liberate the P bound to phytate; thus, the majority of P in plant feed ingredients is not available for absorption, which is the reason for the low digestibility of Ca and P by pigs (Zimmermann et al., 2002; Liao et al., 2005). Therefore, microbial phytase is usually included in diets for pigs to increase P absorption and utilization by hydrolyzing phytic acid within the gastrointestinal tract of pigs (Pallauf et al., 1994). The ability of phytase to increase P digestibility and pig growth performance has been comprehensively described (Selle and Ravindran, 2008; Humer et al., 2015; Holloway et al., 2018).
Pig diets may contain coproducts with high concentration of phytate (e.g., canola meal and rice bran) due to increased availability of these feed ingredients in some areas. Canola meal contains 2.5% to 3.5% phytic acid (Liu et al., 2018), and therefore, high inclusion of canola meal results in increased concentration of phytate in the diets. Phytate from canola meal is also less accessible to phytase compared with phytate from corn and soybean meal (Leske and Coon, 1999). Therefore, the inclusion of phytase at doses above 1,000 phytase units (FTU)/kg may have positive effects on nutrient digestibility and pig growth performance due to increased degradation of phytate in diets (Selle and Ravindran, 2008; Walk et al., 2013; Dersjant-Li and Kwakernaak, 2019).
A next-generation consensus bacterial 6-phytase variant (PhyG; Danisco Animal Nutrition, Leiden, The Netherlands) increased P digestibility in pig diets (Dersjant-Li et al., 2020), but there are limited data to demonstrate the efficacy of this phytase to increase pig growth performance and bone mineralization if included in diets with elevated levels of phytate. Therefore, an experiment was conducted to test the hypothesis that interactions between dietary phytate and dietary concentrations of PhyG exist. The second objective was to test the hypothesis that the use of the novel PhyG phytase results in greater increases in growth performance, bone ash, and apparent total tract digestibility (ATTD) of minerals in diets containing varying phytate concentrations compared with a commercial phytase.
Materials and Methods
The protocol for the experiment was approved by the Institutional Animal Care and Use Committee at the University of Illinois prior to the initiation of the experiment. Pigs that were the offspring of Line 359 boars and Camborough females (Pig Improvement Company, Hendersonville, TN, USA) were used.
Animals and treatments
A total of 144 individually housed growing pigs (initial body weight: 12.70 ± 4.01 kg) were randomly allotted to 18 diets with 8 replicate pigs per diet. Pigs were assigned to treatment groups using a randomized complete block design with 4 blocks of 36 pigs and 2 replicate pigs per diet in each block. The weaning group was used as the blocking factor. Three basal diets that were deficient in P (NRC, 2012) and based on corn, soybean meal, and canola meal (Table 1) were formulated (Table 2). Diets were formulated to contain 0.17% standardized total tract digestible P and to contain 0.23%, 0.29%, or 0.35% phytate-bound P (i.e., low, medium, or high phytate-bound P). Diets were formulated to contain these levels of phytate because most of the commercial diets using soybean meal and canola meal have phytate-bound P within this range, and phytate-bound P in the diets was increased by increasing the concentration of canola meal at the expense of soybean meal and cornstarch. The concentration of monocalcium phosphate in diets containing 0.29% or 0.35% phytate-bound P was also reduced due to increased concentration of P provided by canola meal. Twelve additional diets were formulated by adding 500, 1,000, 2,000, or 4,000 FTU/kg of a novel consensus bacterial 6-phytase variant (PhyG; Danisco Animal Nutrition, the Netherlands) to each of the three basal diets (Tables 3–5). In addition, three reference diets were formulated by adding a commercial Buttiauxella phytase (PhyB; Danisco Animal Nutrition, the Netherlands) at 1,000 FTU/kg to the three basal diets. Thus, a total of 18 diets were used. All diets contained 0.40% titanium dioxide as an indigestible marker. Ingredients were analyzed for the concentration of phytate (Ellis et al., 1977; Eurofins Nutrition Analysis Center, Des Moines, IA, USA) before formulating the diets, and phytate concentration and phytase activity (Method 2000.012; AOAC Int., 2007; Eurofins Nutrition Analysis Center, Des Moines, IA, USA) in all diets were confirmed before starting the animal part of the experiment. Phytase activity in diets was also analyzed and verified using a validated method (ISO 30024:2009; Gizzi et al., 2008; Brabrand, Denmark).
Table 1.
Analyzed nutrient composition of corn, soybean meal, and canola meal, as-fed basis
| Item | Corn | Soybean meal | Canola meal |
|---|---|---|---|
| Dry matter, % | 87.55 | 89.08 | 89.28 |
| Ash, % | 1.28 | 6.41 | 6.84 |
| Gross energy, kcal/kg | 3,857 | 4,208 | 4,272 |
| Crude protein, % | 6.80 | 47.25 | 36.60 |
| AEE1, % | 3.31 | 2.20 | 4.46 |
| Ca, % | 0.01 | 0.33 | 0.77 |
| Total P, % | 0.25 | 0.61 | 1.03 |
| Phytic acid, % | 0.75 | 1.65 | 2.71 |
| Phytate-bound P2, % | 0.21 | 0.47 | 0.76 |
| Non-phytate P3, % | 0.04 | 0.14 | 0.27 |
| Cl, % | <0.10 | <0.10 | <0.10 |
| Na, mg/kg | 10.90 | 26.80 | 676.00 |
| Cu, mg/kg | 1.11 | 13.10 | 4.86 |
| Zn, mg/kg | 15.00 | 34.90 | 43.60 |
| Mn, mg/kg | 3.57 | 28.80 | 57.00 |
| Fe, mg/kg | 12.40 | 83.20 | 204.00 |
| Se, mg/kg | <0.20 | <0.20 | 0.20 |
| Indispensable amino acids, % | |||
| Arg | 0.34 | 3.33 | 2.06 |
| His | 0.21 | 1.23 | 0.98 |
| Ile | 0.26 | 2.28 | 1.54 |
| Leu | 0.76 | 3.60 | 2.55 |
| Lys | 0.27 | 2.96 | 1.97 |
| Met | 0.14 | 0.62 | 0.71 |
| Phe | 0.33 | 2.39 | 1.46 |
| Thr | 0.25 | 1.77 | 1.53 |
| Trp | 0.06 | 0.65 | 0.42 |
| Val | 0.35 | 2.34 | 1.90 |
| Dispensable amino acids, % | |||
| Ala | 0.50 | 2.01 | 1.58 |
| Asp | 0.48 | 5.18 | 2.50 |
| Cys | 0.16 | 0.64 | 0.88 |
| Glu | 1.18 | 8.33 | 6.07 |
| Gly | 0.30 | 1.96 | 1.82 |
| Ser | 0.32 | 1.89 | 1.27 |
| Tyr | 0.17 | 1.69 | 0.98 |
| Pro | 0.57 | 2.28 | 2.22 |
1AEE, acid hydrolyzed ether extract.
2Calculated as 28.2% of phytic acid (Tran and Sauvant, 2004).
3Calculated as total P – phytate-bound P.
Table 2.
Ingredient composition of basal diets containing 0.23%, 0.29%, or 0.35% phytate-bound P, as-fed basis1,2
| Phytate-bound P, % | |||
|---|---|---|---|
| Item | 0.23 | 0.29 | 0.35 |
| Ground corn | 61.08 | 58.05 | 57.28 |
| Soybean meal | 23.00 | 19.00 | 11.90 |
| Canola meal | 1.00 | 12.46 | 23.50 |
| Cornstarch | 9.00 | 5.00 | 2.00 |
| Soybean oil | 3.00 | 3.00 | 3.00 |
| Ground limestone | 1.17 | 1.05 | 0.92 |
| Monocalcium phosphate | 0.17 | 0.05 | — |
| l-Lys | 0.47 | 0.38 | 0.41 |
| dl-Met | 0.06 | 0.02 | — |
| l-Thr | 0.10 | 0.04 | 0.03 |
| l-Trp | — | — | 0.01 |
| Titanium dioxide | 0.40 | 0.40 | 0.40 |
| Salt | 0.40 | 0.40 | 0.40 |
| Vitamin–mineral premix3 | 0.15 | 0.15 | 0.15 |
1Twelve additional diets were prepared by adding a phytase to each basal diet at the expense of corn starch. The phytase premix was prepared by mixing phytase concentrates with corn. The concentrates were formulated to provide 500, 1,000, 2,000, or 4,000 FTU/kg of a novel consensus bacterial 6-phytase variant (PhyG; Danisco Animal Nutrition, The Netherlands).
2Three additional diets were prepared by adding a premix containing a commercial PhyB concentrate and corn to each of the three basal diets. This premix provided 1,000 FTU/kg (PhyB; Danisco Animal Nutrition, The Netherlands) to each diet.
3Provided the following quantities of vitamins and microminerals per kilogram of complete diet: Vitamin A as retinyl acetate, 11,136 IU; vitamin D3 as cholecalciferol, 2,208 IU; vitamin E as dl-alpha tocopheryl acetate, 66 IU; vitamin K as menadione dimethylprimidinol bisulfite, 1.42 mg; thiamin as thiamine mononitrate, 0.24 mg; riboflavin, 6.59 mg; pyridoxine as pyridoxine hydrochloride,0.24 mg; vitamin B12, 0.03 mg; d-pantothenic acid as d-calcium pantothenate, 23.5 mg; niacin, 44.1 mg; folic acid, 1.59 mg; biotin, 0.44 mg; Cu, 20 mg as copper chloride; Fe, 126 mg as ferrous sulfate; I, 1.26 mg as ethylenediamine dihydriodide; Mn, 60.2 mg as manganese hydroxychloride; Se, 0.3 mg as sodium selenite and selenium yeast; and Zn, 125.1 mg as zinc hydroxychloride.
Table 3.
Analyzed nutrient composition of experimental diets containing 0.23% phytate-bound P, as-fed basis
| PhyG, FTU1/kg | PhyB, FTU/kg 1,000 |
|||||
|---|---|---|---|---|---|---|
| Item | 0 | 500 | 1,000 | 2,000 | 4,000 | |
| Dry matter, % | 88.89 | 88.83 | 89.15 | 88.70 | 88.73 | 88.61 |
| Ash, % | 4.20 | 4.23 | 4.22 | 4.17 | 4.23 | 4.39 |
| Gross energy, kcal/kg | 4,009 | 3,994 | 4,031 | 4,003 | 4,021 | 4,009 |
| Crude protein, % | 16.14 | 15.93 | 16.04 | 15.93 | 16.18 | 16.44 |
| AEE1, % | 6.07 | 6.01 | 6.27 | 6.44 | 6.72 | 6.76 |
| Soluble dietary fiber, % | 1.4 | 0.6 | 1.2 | 0.8 | 0.9 | 0.8 |
| Insoluble dietary fiber, % | 10.8 | 10.2 | 10.3 | 10.4 | 10.0 | 10.3 |
| Total dietary fiber, % | 12.2 | 10.8 | 11.5 | 11.2 | 10.9 | 11.1 |
| Phytase, FTU/kg (AOAC) | 100 | 560 | 1,200 | 1,800 | 4,000 | 1,100 |
| Phytase, FTU/kg (ISO 30024:2009) | 242 | 806 | 1,370 | 2,379 | 4,347 | 1,314 |
| Ca, % | 0.61 | 0.64 | 0.62 | 0.66 | 0.71 | 0.69 |
| P, % | 0.32 | 0.33 | 0.33 | 0.33 | 0.35 | 0.36 |
| Phytic acid, % | 0.80 | 0.78 | 0.83 | 0.80 | 0.79 | 0.82 |
| Phytate-bound P2, % | 0.23 | 0.22 | 0.23 | 0.23 | 0.22 | 0.23 |
| Non-phytate P3, % | 0.09 | 0.11 | 0.10 | 0.10 | 0.13 | 0.13 |
| Na, % | 0.16 | 0.16 | 0.16 | 0.16 | 0.17 | 0.16 |
| Mg, % | 0.13 | 0.12 | 0.13 | 0.13 | 0.13 | 0.13 |
| K, % | 0.76 | 0.77 | 0.78 | 0.78 | 0.76 | 0.77 |
| S, % | 0.19 | 0.17 | 0.18 | 0.18 | 0.18 | 0.19 |
| Cl, % | 0.34 | 0.34 | 0.35 | 0.36 | 0.34 | 0.33 |
| Cu, mg/kg | 30.6 | 33.4 | 28.8 | 25.0 | 26.9 | 23.4 |
| Zn, mg/kg | 139 | 138 | 139 | 135 | 143 | 136 |
| Mn, mg/kg | 64.2 | 71.7 | 74.0 | 67.7 | 70.2 | 74.2 |
| Fe, mg/kg | 200 | 225 | 192 | 207 | 182 | 194 |
| Se, mg/kg | 0.55 | 0.50 | 0.46 | 0.45 | 0.50 | 0.49 |
1FTU, phytase units; AEE, acid hydrolyzed ether extract.
2Calculated as 28.2% of phytic acid (Tran and Sauvant, 2004).
3Calculated as total P – phytate-bound P.
Table 5.
Analyzed nutrient composition of experimental diets containing 0.35% phytate-bound P, as-fed basis
| PhyG, FTU1/kg | PhyB1, FTU/kg 1,000 |
|||||
|---|---|---|---|---|---|---|
| Item | 0 | 500 | 1,000 | 2,000 | 4,000 | |
| Dry matter, % | 88.60 | 88.70 | 88.62 | 88.60 | 88.71 | 88.40 |
| Ash, % | 4.41 | 4.44 | 4.81 | 4.71 | 4.75 | 4.78 |
| Gross energy, kcal/kg | 4,102 | 4,096 | 4,110 | 4,102 | 4,110 | 4,089 |
| Crude protein, % | 18.93 | 18.64 | 18.83 | 18.57 | 18.40 | 18.58 |
| AEE1, % | 5.74 | 5.22 | 5.70 | 5.37 | 5.66 | 5.92 |
| Soluble dietary fiber, % | 1.3 | 1.0 | 2.2 | 1.0 | 1.4 | 2.20 |
| Insoluble dietary fiber, % | 13.9 | 14.0 | 14.8 | 14.3 | 15.2 | 13.8 |
| Total dietary fiber, % | 15.2 | 15.0 | 17.0 | 15.3 | 16.6 | 16.0 |
| Phytase, FTU/kg (AOAC) | <70 | 480 | 1,100 | 1,800 | 4,000 | 840 |
| Phytase, FTU/kg (ISO 30024:2009) | 183 | 682 | 1,220 | 2,240 | 4,239 | 1,227 |
| Ca, % | 0.71 | 0.70 | 0.69 | 0.67 | 0.72 | 0.66 |
| P, % | 0.46 | 0.48 | 0.46 | 0.46 | 0.45 | 0.46 |
| Phytic acid, % | 1.22 | 1.20 | 1.24 | 1.22 | 1.20 | 1.22 |
| Phytate-bound P2, % | 0.34 | 0.34 | 0.35 | 0.34 | 0.34 | 0.34 |
| Non-phytate P3, % | 0.12 | 0.14 | 0.11 | 0.12 | 0.11 | 0.12 |
| Na, % | 0.18 | 0.17 | 0.17 | 0.18 | 0.19 | 0.18 |
| Mg, % | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 |
| K, % | 0.75 | 0.73 | 0.74 | 0.72 | 0.73 | 0.75 |
| S, % | 0.31 | 0.29 | 0.30 | 0.32 | 0.31 | 0.32 |
| Cl, % | 0.35 | 0.33 | 0.33 | 0.34 | 0.35 | 0.34 |
| Cu, mg/kg | 25.1 | 26.4 | 24.1 | 25.3 | 24.5 | 31.0 |
| Zn, mg/kg | 146 | 143 | 145 | 144 | 147 | 145 |
| Mn, mg/kg | 84.9 | 81.3 | 76.1 | 77.9 | 76.5 | 77.5 |
| Fe, mg/kg | 209 | 200 | 197 | 221 | 210 | 225 |
| Se, mg/kg | 0.78 | 0.70 | 0.77 | 0.84 | 0.80 | 0.75 |
1FTU, phytase units; AEE, acid hydrolyzed ether extract.
2Calculated as 28.2% of phytic acid (Tran and Sauvant, 2004).
3Calculated as total P – phytate-bound P.
Table 4.
Analyzed nutrient composition of experimental diets containing 0.29% phytate-bound P, as-fed basis
| PhyG, FTU1/kg | PhyB, FTU/kg 1,000 |
|||||
|---|---|---|---|---|---|---|
| Item | 0 | 500 | 1,000 | 2,000 | 4,000 | |
| Dry matter, % | 88.57 | 88.40 | 88.49 | 88.78 | 88.68 | 88.45 |
| Ash, % | 4.59 | 4.64 | 4.63 | 4.77 | 4.67 | 4.59 |
| Gross energy, kcal/kg | 4,067 | 4,056 | 4,057 | 4,065 | 4,050 | 4,057 |
| Crude protein, % | 17.92 | 18.03 | 18.43 | 18.20 | 17.78 | 17.66 |
| AEE1, % | 5.43 | 5.99 | 6.06 | 6.09 | 5.44 | 5.50 |
| Soluble dietary fiber, % | 0.6 | 0.9 | 0.8 | 0.8 | 1.0 | 0.7 |
| Insoluble dietary fiber, % | 13.1 | 12.1 | 12.0 | 14.1 | 12.1 | 12.2 |
| Total dietary fiber, % | 13.7 | 13.0 | 12.8 | 14.9 | 13.1 | 12.9 |
| Phytase, FTU/kg (AOAC) | <70 | 470 | 840 | 2,100 | 4,200 | 810 |
| Phytase, FTU/kg (ISO 30024:2009) | 188 | 696 | 1,309 | 2,406 | 4,680 | 1,369 |
| Ca, % | 0.65 | 0.69 | 0.66 | 0.70 | 0.71 | 0.69 |
| P, % | 0.40 | 0.40 | 0.42 | 0.40 | 0.43 | 0.43 |
| Phytic acid, % | 1.05 | 1.04 | 1.01 | 1.03 | 1.00 | 1.04 |
| Phytate-bound P2, % | 0.30 | 0.29 | 0.28 | 0.29 | 0.28 | 0.29 |
| Non-phytate P3, % | 0.10 | 0.11 | 0.14 | 0.11 | 0.15 | 0.14 |
| Na, % | 0.17 | 0.17 | 0.17 | 0.17 | 0.16 | 0.17 |
| Mg, % | 0.18 | 0.17 | 0.18 | 0.18 | 0.17 | 0.18 |
| K, % | 0.80 | 0.76 | 0.79 | 0.81 | 0.79 | 0.79 |
| S,% | 0.25 | 0.22 | 0.25 | 0.26 | 0.25 | 0.26 |
| Cl, % | 0.32 | 0.32 | 0.31 | 0.33 | 0.32 | 0.32 |
| Cu, mg/kg | 23.9 | 26.1 | 30.2 | 25.1 | 34.4 | 26.7 |
| Zn, mg/kg | 141.0 | 136 | 144 | 152 | 144 | 138 |
| Mn, mg/kg | 76.3 | 69.1 | 71.3 | 81.4 | 71.7 | 74 |
| Fe, mg/kg | 189 | 194 | 196 | 191 | 198 | 191 |
| Se, mg/kg | 0.64 | 0.61 | 0.67 | 0.64 | 0.61 | 0.60 |
1FTU, phytase units; AEE, acid hydrolyzed ether extract.
2Calculated as 28.2% of phytic acid (Tran and Sauvant, 2004).
3Calculated as total P – phytate-bound P.
Experimental procedures
Pig weights were recorded at the beginning and at the conclusion of the experiment. The initial 15 d was considered the adaptation period to the experimental diets. During the adaptation period, pigs had free access to feed and water. However, from day 16 to 20, pigs were limit-fed in a daily amount of 3.2 times the estimated metabolizable energy requirement for maintenance (i.e., 197 kcal/kg × body weight0.60; NRC, 2012). Feed addition was recorded daily, and the weight of non-consumed feed was recorded. Water was available at all times.
Sample collection
Fecal samples were collected via anal stimulation on days 16, 17, 18, and 19, and collected samples were stored at −20 °C. On the last day of the experiment (day 20), pigs were weighed, feeders were emptied, and the amount of feed left in each feeder was recorded and subtracted from total feed allotments to calculate feed disappearance for each pig. Four hours after feeders had been emptied, pigs were euthanized and the right femur was collected.
Chemical analyses
At the conclusion of the experiment, fecal samples were dried in a forced-air drying oven at 50 °C. Fecal samples were finely ground through a 1-mm screen in a Wiley mill (Model 4; Thomas Scientific, Swedesboro, NJ, USA) before analysis. Ingredients, diets, and fecal samples were analyzed for dry matter (method 930.15; AOAC Int., 2007). Diets and fecal samples were analyzed for titanium (Myers et al., 2004). Ingredients, diets, and fecal samples were analyzed for minerals by inductively coupled plasma optical emission spectrometry using an internally validated method (method 985.01 A, B, and C; AOAC Int., 2007) after wet ash sample preparation [method 975.03 B(b); AOAC Int., 2007]. Ingredients were analyzed for amino acids on a Hitachi amino acid analyzer (Model No. L8800; Hitachi High Technologies America, Inc., Pleasanton, CA, USA) using ninhydrin for post-column derivatization and norleucine as the internal standard. Diets and ingredients were analyzed for ash (method 942.05; AOAC Int., 2007), and crude protein was calculated as analyzed concentration of nitrogen multiplied by 6.25. Nitrogen was measured using the combustion procedure (method 990.03; AOAC Int., 2007) on a LECO FP628 (LECO Corp., Saint Joseph, MI, USA). Diets and ingredients were also analyzed for acid-hydrolyzed ether extract (AEE) by acid hydrolysis using 3N HCl (AnkomHCl, Ankom Technology, Macedon, NY, USA) followed by fat extraction using petroleum ether (AnkomXT15, Ankom Technology, Macedon, NY, USA). Gross energy was analyzed in diets and ingredients on an isoperibol bomb calorimeter (Model 6400, Parr Instruments, Moline, IL, USA) using benzoic acid as the internal standard. Diets were analyzed for insoluble dietary fiber and soluble dietary fiber according to method 991.43 (AOAC Int., 2007) using the AnkomTDF Dietary Fiber Analyzer (Ankom Technology, Macedon, NY, USA). Total dietary fiber was calculated as the sum of insoluble dietary fiber and soluble dietary fiber. Femurs were autoclaved at 125 °C for 55 min and cleaned for soft tissue. Femurs were broken, dried, and soaked for 72 h in petroleum ether under a chemical hood to remove marrow and fat. Femurs were dried for 2 h at 135 °C and then ashed at 600 °C for 16 h. Bone ash samples were analyzed for Ca and P via inductively coupled plasma optical emission spectrometry. Analyses for amino acids and minerals were conducted at the Agricultural Experiment Station Chemical Laboratories at the University of Missouri-Columbia (Columbia, MO, USA), and all other analyses were conducted in the Monogastric Nutrition Laboratory at the University of Illinois at Urbana-Champaign (Urbana, IL, USA).
Calculations and statistical analysis
Values for the ATTD of minerals in all diets were calculated (Adeola, 2001). Concentrations of bone Ca and bone P in grams per femur were calculated by multiplying the total quantity of bone ash by the percentage of Ca and P in bone ash. Growth performance data were summarized for the entire experiment to calculate average daily feed intake (ADFI), average daily gain (ADG), and gain to feed ratio (G:F) within each pen and treatment group.
Normality of residuals was verified using the UNIVARIATE procedure (SAS Inst. Inc., Cary, NC) and influence of options of SAS. Outliers were identified and removed as values that deviated from the treatment mean by more than 1.5 times the interquartile range. For growth performance data, no outliers were identified. For mineral digestibility, removed outliers included one pig fed each of the following diets: 0.23% phytate-bound P with 4,000 FTU/kg PhyG; 0.35% phytate-bound P with 1,000 FTU/kg PhyG; 0.23% phytate-bound P with 1,000 FTU/kg PhyB;, 0.29% phytate-bound P with 1,000 FTU/kg PhyB; and 0.35% phytate-bound P with 4,000 FTU/kg PhyG. One pig fed the diet containing 0.35% phytate-bound P and no phytase was also identified as an outlier and removed from data for bone parameters. Data were analyzed as a randomized complete block design in a 3 × 5 factorial arrangement with the pig as the experimental unit. The model included amount of phytate-bound P, phytase (i.e., PhyG), and the interaction between phytate-bound P and PhyG as the main effects. Block and replicate within block were considered random effects. Linear and quadratic effects of increasing levels of PhyG were determined for each phytate level using orthogonal CONTRAST statements. Contrast statements were used with coefficients for unequally spaced treatments being generated using the Proc Interactive Matrix Language statement in SAS. Contrast statements were used to determine the effects of PhyG on mineral digestibility, concentrations of apparent total tract digestible minerals, bone ash, and pig growth performance by comparing results for pigs fed diets containing 1,000 FTU/kg of PhyG with results for pigs fed diets supplemented with 1,000 FTU/kg from PhyB. Treatment means were calculated and separated using the LSMEANS statement and the PDIFF option of PROC MIXED, respectively. Regression equations for the concentration of apparent total tract digestible P as a function of PhyG dose were developed using the exponential curve fitting of JMP 14.0 (SAS Institute Inc., Cary, NC). Statistical significance and tendencies were considered at P < 0.05 and 0.05 ≤ P < 0.10, respectively.
Results
Diet analyses indicated that the intended concentrations of phytate and phytase were present in all diets. Concentrations of other nutrients were not affected by dietary treatment, and pigs consumed their diets without apparent problems. Although diet and fecal samples were analyzed for both macro- and micro-minerals, results obtained for micro-minerals were erratic and are not reported.
No interactions between phytate and phytase were observed for the growth performance of pigs (Table 6). Average daily gain, ADFI, G:F, and final body weight of pigs increased (P < 0.01) when PhyG was included in the diets. Pigs fed diets containing 0.29% or 0.35% phytate-bound P also had greater (P < 0.01) ADG, ADFI, and final body weight compared with pigs fed the diet containing 0.23% phytate-bound P. Pigs fed the 0.29% phytate-bound P diet tended to have greater (P < 0.10) G:F compared with pigs fed the 0.23% phytate-bound P diet. Linear and/or quadratic increases (P < 0.05) were observed for ADG, ADFI, G:F, and final body weight of pigs as PhyG increased in diets containing 0.23%, 0.29%, or 0.35% phytate-bound P.
Table 6.
Overall growth performance of pigs fed diets containing different concentrations of phytate and phytase dose1,2
| Item | Initial body weight, kg | ADG, kg | ADFI, kg | G:F | Final body weight, kg |
|---|---|---|---|---|---|
| 0.23% phytate-bound P3 | |||||
| 0 PhyG, FTU/kg | 12.67 | 0.375 | 0.839 | 0.441 | 20.18 |
| 500 PhyG, FTU/kg | 12.35 | 0.511 | 0.965 | 0.530 | 22.56 |
| 1,000 PhyG, FTU/kg | 12.21 | 0.571 | 1.003 | 0.569 | 23.62 |
| 2,000 PhyG, FTU/kg | 12.65 | 0.533 | 0.961 | 0.559 | 23.31 |
| 4,000 PhyG, FTU/kg | 12.47 | 0.560 | 0.975 | 0.574 | 23.67 |
| 0.29% phytate-bound P4 | |||||
| 0 PhyG, FTU/kg | 12.99 | 0.442 | 0.921 | 0.479 | 21.82 |
| 500 PhyG, FTU/kg | 12.90 | 0.589 | 1.051 | 0.559 | 24.69 |
| 1,000 PhyG, FTU/kg | 12.66 | 0.606 | 1.023 | 0.596 | 24.78 |
| 2,000 PhyG, FTU/kg | 12.79 | 0.605 | 1.045 | 0.578 | 24.89 |
| 4,000 PhyG, FTU/kg | 12.44 | 0.593 | 0.987 | 0.604 | 24.30 |
| 0.35% phytate-bound P5 | |||||
| 0 PhyG, FTU/kg | 12.72 | 0.457 | 0.959 | 0.479 | 21.86 |
| 500 PhyG, FTU/kg | 12.91 | 0.543 | 0.986 | 0.551 | 23.77 |
| 1,000 PhyG, FTU/kg | 13.16 | 0.624 | 1.081 | 0.576 | 25.64 |
| 2,000 PhyG, FTU/kg | 13.18 | 0.626 | 1.071 | 0.587 | 25.68 |
| 4,000 PhyG, FTU/kg | 12.86 | 0.589 | 1.040 | 0.563 | 24.63 |
| SEM | 1.43 | 0.04 | 0.06 | 0.02 | 2.01 |
| P-value | |||||
| Phytate-bound P | 0.580 | 0.004 | 0.004 | 0.075 | <0.001 |
| PhyG | 0.993 | <0.001 | <0.001 | <0.001 | <0.001 |
| Phytate-bound P × PhyG | 0.999 | 0.824 | 0.733 | 0.944 | 0.881 |
1Data are least squares means of eight observations per treatment.
2ADG, average daily gain; ADFI, average daily feed intake; G:F, gain to feed ratio; FTU, phytase units.
3Linear and quadratic increase for ADG, G:F, and final body weight: P < 0.05; Quadratic increase for ADFI: P < 0.05.
4Linear and quadratic increase for ADG and G:F: P < 0.05; Quadratic increase for ADFI and final body weight: P < 0.05.
5Linear and quadratic increase for ADG, G:F, and final body weight: P < 0.05; Quadratic increase for ADFI: P < 0.05.
Diets containing 0.35% phytate-bound P had reduced (P < 0.01) the ATTD of Ca, P, Mg, and K compared with diets containing 0.23% or 0.29% phytate-bound P, but the inclusion of PhyG to diets increased (P < 0.01) the ATTD of Ca, Na, and K (Table 7). PhyG increased the ATTD of P and Mg, but to a greater extent in diets with 0.23% or 0.29% phytate-bound P, than in diets with 0.35% phytate-bound P (interaction, P < 0.05). Regardless of phytate level in the diets, increasing levels of PhyG increased (linear and quadratic, P < 0.05) the ATTD of Ca, P, Na, and K. Increasing levels of PhyG linearly increased (P < 0.05) the ATTD of Mg in diets with 0.23% phytate-bound P.
Table 7.
Apparent total tract digestibility of minerals in diets containing different concentrations of phytate and phytase dose1,2
| Apparent total tract digestibility, % | |||||
|---|---|---|---|---|---|
| Item | Ca | P | Na | Mg | K |
| 0.23% phytate-bound P3 | |||||
| 0 PhyG, FTU/kg | 61.2 | 21.2f | 74.0 | 21.0defg | 76.9 |
| 500 PhyG, FTU/kg | 78.5 | 56.4d | 81.5 | 22.6def | 81.3 |
| 1,000 PhyG, FTU/kg | 82.3 | 67.9bc | 87.5 | 29.6abc | 82.0 |
| 2,000 PhyG, FTU/kg | 83.8 | 68.9bc | 89.6 | 30.7ab | 82.6 |
| 4,000 PhyG, FTU/kg | 83.3 | 74.7a | 91.6 | 32.2a | 82.7 |
| 0.29% phytate-bound P4 | |||||
| 0 PhyG, FTU/kg | 56.2 | 23.4f | 76.2 | 17.7fgh | 73.9 |
| 500 PhyG, FTU/kg | 74.3 | 51.2e | 76.2 | 24.8bcd | 74.5 |
| 1,000 PhyG, FTU/kg | 74.5 | 59.6d | 85.3 | 21.1defg | 74.6 |
| 2,000 PhyG, FTU/kg | 80.5 | 72.1ab | 89.5 | 25.1bcd | 80.1 |
| 4,000 PhyG, FTU/kg | 79.4 | 74.4a | 92.6 | 24.0cde | 82.6 |
| 0.35% phytate-bound P5 | |||||
| 0 PhyG, FTU/kg | 54.6 | 24.1f | 68.9 | 16.2gh | 69.6 |
| 500 PhyG, FTU/kg | 69.7 | 48.6e | 76.8 | 18.5efgh | 67.3 |
| 1,000 PhyG, FTU/kg | 73.7 | 60.1d | 83.3 | 21.5defg | 71.5 |
| 2,000 PhyG, FTU/kg | 71.6 | 66.9c | 92.1 | 16.5gh | 76.9 |
| 4,000 PhyG, FTU/kg | 73.8 | 66.9bc | 91.1 | 14.8h | 71.0 |
| SEM | 2.67 | 2.50 | 2.83 | 3.52 | 2.00 |
| P-value | |||||
| Phytate-bound P | <0.001 | <0.001 | 0.242 | <0.001 | <0.001 |
| PhyG | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 |
| Phytate-bound P × PhyG | 0.735 | 0.002 | 0.419 | 0.014 | 0.062 |
1FTU, phytase units.
2Data are least squares means of eight observations per treatment, except for 0.23% phytate-bound P with 4,000 FTU/kg PhyG diet, 0.35% phytate-bound P with 1,000 FTU/kg PhyG diet, 0.23% phytate-bound P with 1,000 FTU/kg PhyB diet, 0.29% phytate-bound P with 1,000 FTU/kg PhyB diet, and 0.35% phytate-bound P with 4,000 FTU/kg PhyG diet which represent seven observations per treatment.
3Linear and quadratic increase for ATTD of Ca, P, Na, Mg: P < 0.05; Linear increase for ATTD of K: P < 0.05.
4Linear and quadratic increase for ATTD of Ca, P, and Na: P < 0.05; Linear increase for ATTD of K: P < 0.05.
5Linear and quadratic increase for ATTD of Ca, P, and Na: P < 0.05; Quadratic increase for ATTD of K: P < 0.05.
a–hMeans within a column lacking a common letter are different (P ≤ 0.05).
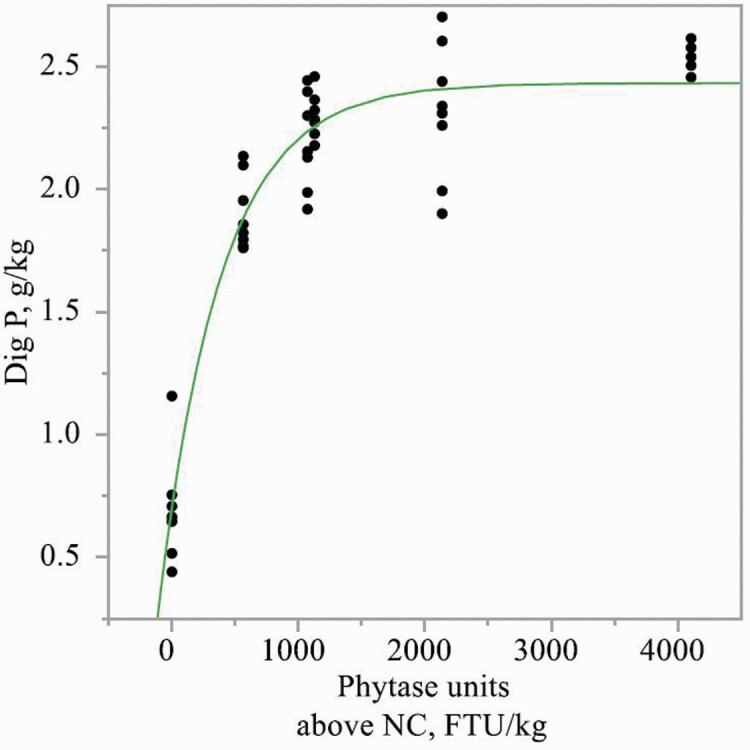
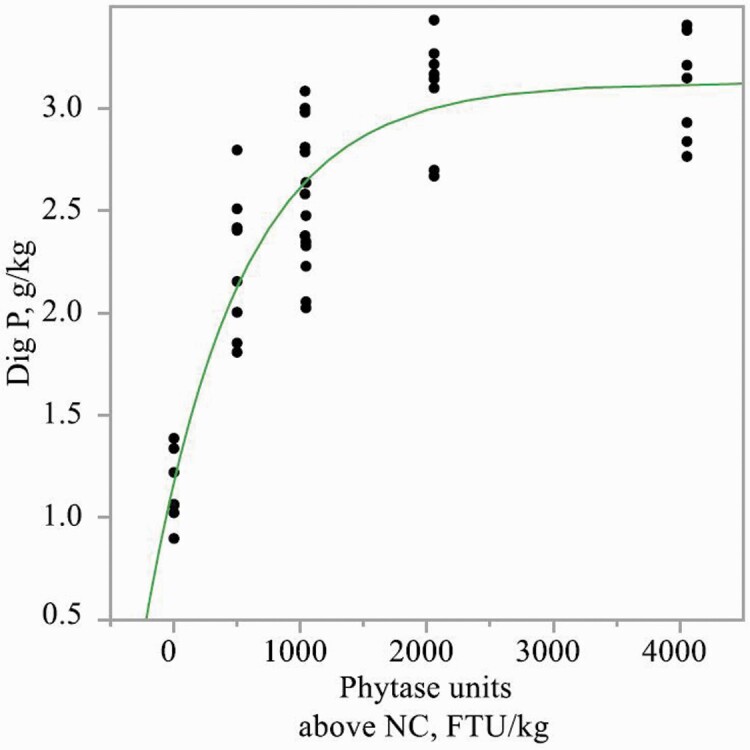
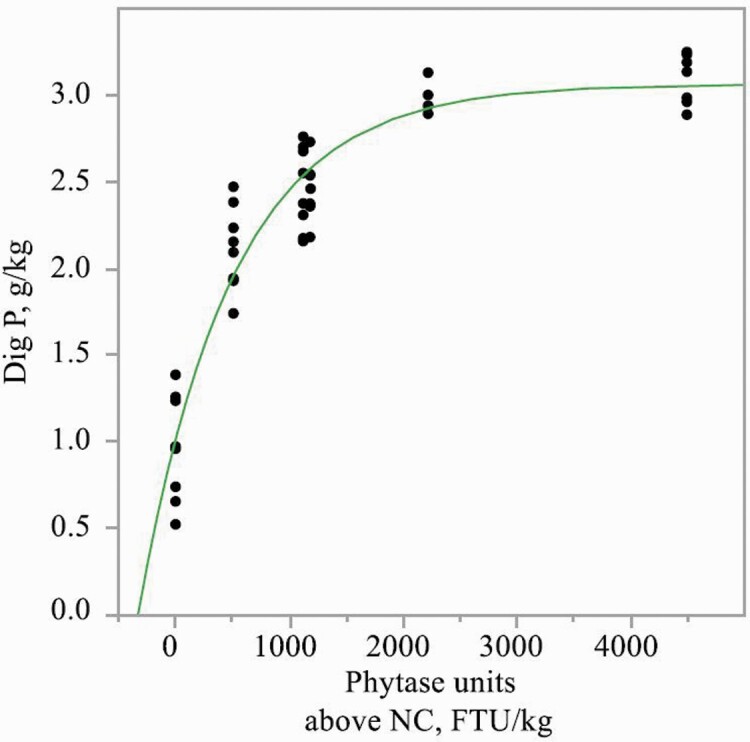
Inclusion of PhyG to diets increased (P < 0.01) the concentrations of apparent total tract digestible Ca, K, and Mg, but to a greater extent in diets with 0.23% or 0.29% phytate-bound P than in diets with 0.35% phytate-bound P (interaction, P < 0.05; Table 8). PhyG also increased concentrations of apparent total tract digestible P and Na, but to a greater extent in diets with 0.29% or 0.35% phytate-bound P, than in diets with 0.23% phytate-bound P (interaction, P < 0.05). PhyG increased the concentration of apparent total tract digestible P (exponential, P < 0.01) in diets containing 0.23%, 0.29%, or 0.35% phytate-bound P (Figures 1–3). Linear and/or quadratic increases (P < 0.05) were observed for concentrations of apparent total tract digestible Ca, P, Na, and K as PhyG increased in diets containing 0.23%, 0.29%, or 0.35% phytate-bound P. Increasing levels of PhyG linearly increased (P < 0.05) apparent total tract digestible Mg in diets with 0.23% phytate-bound P.
Table 8.
Concentrations (g/kg) of apparent total tract digestible minerals in diets containing different concentrations of phytate and phytase dose1–3
| Apparent total tract digestible minerals, g/kg | |||||
|---|---|---|---|---|---|
| Item | Ca | P | Na | Mg | K |
| 0.23% phytate-bound P4 | |||||
| 0 PhyG, FTU/kg | 3.70f | 0.68j | 1.16g | 0.27e | 5.85c |
| 500 PhyG, FTU/kg | 5.05de | 1.88h | 1.27fg | 0.28e | 6.22ab |
| 1,000 PhyG, FTU/kg | 5.08de | 2.26fg | 1.39de | 0.37abcde | 6.36a |
| 2,000 PhyG, FTU/kg | 5.54bc | 2.29f | 1.43cde | 0.38abcde | 6.41a |
| 4,000 PhyG, FTU/kg | 5.95a | 2.63de | 1.52bc | 0.40abcd | 6.28a |
| 0.29% phytate-bound P5 | |||||
| 0 PhyG, FTU/kg | 3.66f | 0.94i | 1.27fg | 0.31de | 5.89bc |
| 500 PhyG, FTU/kg | 5.14cde | 2.07g | 1.27fg | 0.43ab | 5.69c |
| 1,000 PhyG, FTU/kg | 4.92de | 2.52e | 1.48bcd | 0.37abcde | 5.89bc |
| 2,000 PhyG, FTU/kg | 5.67ab | 2.90bc | 1.54b | 0.45ab | 6.48a |
| 4,000 PhyG, FTU/kg | 5.61ab | 3.23a | 1.52bc | 0.42abc | 6.50a |
| 0.35% phytate-bound P6 | |||||
| 0 PhyG, FTU/kg | 3.88f | 1.12i | 1.21g | 0.35bcde | 5.19e |
| 500 PhyG, FTU/kg | 4.86e | 2.32f | 1.34ef | 0.39abcd | 4.90e |
| 1,000 PhyG, FTU/kg | 5.07de | 2.76cd | 1.43bcde | 0.46a | 5.27de |
| 2,000 PhyG, FTU/kg | 4.80e | 3.06ab | 1.69a | 0.35bcde | 5.56cd |
| 4,000 PhyG, FTU/kg | 5.30bcd | 3.01b | 1.73a | 0.31cde | 5.20e |
| SEM | 0.179 | 0.102 | 0.047 | 0.065 | 0.153 |
| P-value | |||||
| Phytate-bound P | 0.007 | <0.001 | <0.001 | 0.066 | <0.001 |
| PhyG | <0.001 | <0.001 | <0.001 | 0.041 | <0.001 |
| Phytate-bound P × PhyG | 0.005 | <0.001 | 0.004 | 0.050 | 0.017 |
1FTU, phytase units.
2Data are least squares means of eight observations per treatment, except for 0.23% phytate-bound P with 4,000 FTU/kg PhyG diet, 0.35% phytate-bound P with 1,000 FTU/kg PhyG diet, 0.23% phytate-bound P with 1,000 FTU/kg PhyB diet, 0.29% phytate-bound P with 1,000 FTU/kg PhyB diet, and 0.35% phytate-bound P with 4,000 FTU/kg PhyG diet which represent seven observations per treatment.
3Concentrations of apparent total tract digestible minerals in the diets were calculated by multiplying values for the ATTD (%) of minerals by the analyzed concentration of minerals in the diets.
4Linear and quadratic increase for Ca, P, and Na: P < 0.05; Linear increase for Mg: P < 0.05; Quadratic increase for K: P < 0.05.
5Linear and quadratic increase for Ca, P, and Na: P < 0.05; Linear increase for K: P < 0.05.
6Linear and quadratic increase for Ca, P, and Na: P < 0.05; Quadratic increase for K: P < 0.05.
a–jMeans within a column lacking a common letter are different (P ≤ 0.05).
Figure 1.
Exponential model of concentration of apparent total tract digestible P in diets containing 0.23% phytate-bound P as a function of PhyG concentration. Digestible P, g/kg = 2.43 − 1.73 × [exp (−0.002 × phytase dose in FTU/kg)]; R2 = 0.91.
Figure 3.
Exponential model of concentration of apparent total tract digestible P in diets containing 0.35% phytate-bound P as a function of PhyG concentration. Digestible P, g/kg = 3.12 − 1.95 × [exp (−0.001 × phytase dose in FTU/kg)]; R2 = 0.83.
Figure 2.
Exponential model of concentration of apparent total tract digestible P in diets containing 0.29% phytate-bound P as a function of PhyG concentration. Digestible P, g/kg = 3.06 − 2.05 × [exp (−0.001 × phytase dose in FTU/kg)]; R2 = 0.91.
Inclusion of PhyG to diets increased bone ash (expressed as grams per femur and %), bone Ca (g per femur), and bone P (g per femur) of pigs, but to a greater extent if there was 0.35% or 0.29% rather than 0.23% phytate-bound P in the diets (interaction, P < 0.01; Table 9). Inclusion of PhyG to diets resulted in an increased (P < 0.01) concentration of bone P (%) and, therefore, reduced (P < 0.01) Ca:P in the bone of pigs. Pigs fed diets with 0.35% or 0.29% phytate-bound P also had greater (P < 0.01) concentration of bone P (%) and reduced (P < 0.01) bone Ca:P compared with pigs fed diets with 0.23% phytate-bound P. Increasing levels of PhyG increased (linear and quadratic, P < 0.05) bone ash (% and g per femur), bone Ca (g per femur), and bone P (g per femur) in diets regardless of dietary phytate.
Table 9.
Concentrations (g per femur) and percentage of bone ash, bone Ca, and bone P in pigs fed diets containing different concentrations of phytate and phytase dose1,2
| Item | Bone ash, g per femur | Bone Ca, g per femur | Bone P, g per femur | Bone ash, % | Bone Ca, % | Bone P, % | Ca:P in bone |
|---|---|---|---|---|---|---|---|
| 0.23% phytate-bound P3 | |||||||
| 0 PhyG, FTU/kg | 7.52f | 2.85f | 1.34g | 45.55g | 38.15 | 17.87 | 2.14 |
| 500 PhyG, FTU/kg | 10.72e | 4.17e | 2.00f | 51.84ef | 39.02 | 18.75 | 2.08 |
| 1,000 PhyG, FTU/kg | 11.26de | 4.37de | 2.09f | 53.18bcd | 38.74 | 18.61 | 2.08 |
| 2,000 PhyG, FTU/kg | 12.76bc | 4.96c | 2.39cd | 53.99abc | 38.90 | 18.72 | 2.09 |
| 4,000 PhyG, FTU/kg | 12.67bcd | 4.84cd | 2.34de | 52.45def | 38.48 | 18.55 | 2.07 |
| 0.29% phytate-bound P4 | |||||||
| 0 PhyG, FTU/kg | 7.74f | 2.92f | 1.30g | 43.20h | 38.48 | 18.46 | 2.10 |
| 500 PhyG, FTU/kg | 11.55cde | 4.43de | 2.15ef | 51.41f | 38.50 | 18.68 | 2.06 |
| 1,000 PhyG, FTU/kg | 12.53bcd | 5.20bc | 2.56bcd | 52.81cde | 38.91 | 19.15 | 2.03 |
| 2,000 PhyG, FTU/kg | 13.83ab | 5.33abc | 2.61bc | 54.37ab | 38.63 | 18.89 | 2.04 |
| 4,000 PhyG, FTU/kg | 14.31a | 5.55ab | 2.70ab | 54.63a | 38.87 | 18.95 | 2.05 |
| 0.35% phytate-bound P5 | |||||||
| 0 PhyG, FTU/kg | 6.85f | 2.62f | 1.25g | 43.42h | 38.38 | 18.13 | 2.09 |
| 500 PhyG, FTU/kg | 11.45cde | 4.42de | 2.12ef | 51.39f | 38.86 | 18.64 | 2.09 |
| 1,000 PhyG, FTU/kg | 14.76a | 5.73a | 2.73ab | 54.03abc | 38.90 | 18.79 | 2.07 |
| 2,000 PhyG, FTU/kg | 14.94a | 5.80a | 2.84a | 54.47a | 38.91 | 19.06 | 2.04 |
| 4,000 PhyG, FTU/kg | 14.39a | 5.50ab | 2.71ab | 54.05abc | 38.38 | 18.89 | 2.02 |
| SEM | 1.00 | 0.32 | 0.15 | 0.63 | 0.71 | 0.32 | 0.01 |
| P-value | |||||||
| Phytate-bound P | <0.001 | <0.001 | <0.001 | 0.820 | 0.995 | 0.008 | <0.001 |
| PhyG | <0.001 | <0.001 | <0.001 | <0.001 | 0.394 | <0.001 | <0.001 |
| Phytate-bound P × PhyG | 0.009 | 0.004 | 0.003 | <0.001 | 0.932 | 0.440 | 0.095 |
1Data are least squares means of eight observations per treatment, except for 0.35% phytate-bound P without PhyG diet which represents seven observations per treatment.
2FTU, phytase units.
3Linear and quadratic increase for bone ash (% and g per femur), bone Ca (g per femur), and bone P (g per femur): P < 0.05; Quadratic increase for bone P (%): P < 0.05; Linear increase for Ca:P in bone: P < 0.05.
4Linear and quadratic increase for bone ash (% and g per femur), bone Ca (g per femur), and bone P (g per femur): P < 0.05; Tendency for linear and quadratic increase for bone P (%): P < 0.10; Quadratic increase for Ca:P in bone: P < 0.05.
5Linear and quadratic increase for bone ash (% and g per femur), bone Ca (g per femur), and bone P (g per femur): P < 0.05; Linear and quadratic increase for bone P (%): P < 0.05; Linear increase for Ca:P in bone: P < 0.05.
a–hMeans within a column lacking a common letter are different (P ≤ 0.05).
At 0.23% phytate-bound P, pigs fed diets containing 1,000 FTU/kg PhyG tended to have greater (P < 0.10) ADG compared with pigs fed diets containing PhyB (Table 10). Overall, pigs fed diets containing PhyB tended to have reduced (P < 0.10) ADG and ADFI compared with pigs fed diets containing PhyG (1,000 FTU/kg). At 0.35% phytate-bound P, diets containing 1,000 FTU/kg PhyG had greater (P < 0.05) ATTD of Ca and P compared with pigs fed diets containing PhyB. As a result, diets containing 1,000 FTU/kg PhyG had greater ATTD of P (P < 0.05) than diets containing 1,000 FTU/kg PhyB. Overall, pigs fed diets containing PhyG (1,000 FTU/kg) had greater (P < 0.01) bone ash (%), bone Ca (g per femur), and bone P (g per femur) compared with pigs fed diets containing PhyB.
Table 10.
Effects of PhyG and PhyB on growth performance, apparent total tract digestibility of minerals, and bone parameters of growing pigs1
| 0.23% phytate-P | 0.29% phytate-P | 0.35% phytate-P | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | PhyG | PhyB | PhyG | PhyB | PhyG | PhyB | SEM | 0.23% phytate-P | 0.29% phytate-P | 0.35% phytate-P | Overall2 |
| Growth performance, day 1 to 20 | |||||||||||
| Initial body weight, kg | 12.21 | 12.19 | 12.66 | 12.37 | 13.16 | 13.17 | 1.43 | 0.977 | 0.505 | 0.982 | 0.698 |
| ADG, kg | 0.571 | 0.510 | 0.606 | 0.579 | 0.624 | 0.594 | 0.04 | 0.098 | 0.450 | 0.404 | 0.062 |
| ADFI, kg | 1.003 | 0.921 | 1.023 | 0.990 | 1.081 | 1.044 | 0.06 | 0.121 | 0.532 | 0.483 | 0.097 |
| G:F | 0.569 | 0.552 | 0.596 | 0.586 | 0.576 | 0.569 | 0.02 | 0.531 | 0.723 | 0.796 | 0.475 |
| Final body weight, kg | 23.62 | 22.40 | 24.78 | 23.94 | 25.64 | 25.04 | 2.02 | 0.195 | 0.371 | 0.525 | 0.104 |
| ATTD of minerals, % | |||||||||||
| Ca | 82.3 | 80.2 | 74.5 | 76.4 | 73.7 | 67.4 | 2.64 | 0.485 | 0.532 | 0.038 | 0.213 |
| P | 67.9 | 64.5 | 59.6 | 58.8 | 60.1 | 50.1 | 2.51 | 0.159 | 0.739 | <0.001 | 0.001 |
| Na | 87.5 | 83.6 | 85.3 | 86.5 | 83.3 | 80.5 | 3.02 | 0.232 | 0.710 | 0.387 | 0.332 |
| Mg | 29.6 | 32.1 | 21.1 | 19.4 | 21.5 | 16.8 | 3.54 | 0.447 | 0.579 | 0.113 | 0.466 |
| K | 82.0 | 82.7 | 74.6 | 76.4 | 71.5 | 69.4 | 2.01 | 0.784 | 0.466 | 0.410 | 0.942 |
| Bone parameters | |||||||||||
| Bone ash, g per femur | 11.26 | 11.22 | 12.53 | 11.78 | 14.76 | 13.51 | 1.02 | 0.951 | 0.280 | 0.096 | 0.102 |
| Bone Ca, g per femur | 4.37 | 4.36 | 5.20 | 4.48 | 5.73 | 5.09 | 0.32 | 0.991 | 0.007 | 0.012 | 0.003 |
| Bone P, g per femur | 2.09 | 2.09 | 2.56 | 2.17 | 2.73 | 2.45 | 0.15 | 0.981 | 0.002 | 0.031 | 0.002 |
| Bone ash, % | 53.18 | 51.74 | 52.81 | 51.79 | 54.03 | 53.46 | 0.59 | 0.019 | 0.117 | 0.346 | 0.006 |
| Bone Ca, % | 38.74 | 38.97 | 38.91 | 38.22 | 38.90 | 37.99 | 0.76 | 0.667 | 0.196 | 0.088 | 0.138 |
| Bone P, % | 18.61 | 18.66 | 19.15 | 18.50 | 18.79 | 18.58 | 0.35 | 0.846 | 0.009 | 0.379 | 0.055 |
| Ca:P in bone | 2.08 | 2.09 | 2.03 | 2.06 | 2.07 | 2.05 | 0.01 | 0.637 | 0.074 | 0.138 | 0.603 |
1Data are least squares means of eight observations per treatment; both phytases were included at 1,000 phytase units/kg.
2Overall: P-value for a contrast analysis conducted between PhyG and PhyB regardless of the level of phytate in the diet.
Discussion
Phytase is an enzyme that hydrolyzes the ester bond between inositol and P in phytate and increases P digestibility by rendering phytate-bound P available for absorption (Jongbloed et al., 1992). However, the efficacy of phytase in increasing nutrient digestibility and pig growth performance may be influenced by phytase source, dietary concentration of phytate, and diet composition (Bedford and Schulze, 1998; Dias et al., 2010). Therefore, one of the objectives of this experiment was to determine the interactive effects of dietary phytate and phytase on nutrient digestibility, bone ash, and growth performance of pigs.
In the present experiment, all basal diets were formulated to be deficient in standardized total tract digestible P to test the hypothesis that the inclusion of phytase will improve growth performance of pigs. The observed increase in ADG, ADFI, G:F, and final body weight as increased concentrations of phytase were included in the diets confirms this hypothesis, and this observation concurs with previous data (Braña et al., 2006). The increased mineral digestibility upon phytase supplementation resulted in increased availability of digestible minerals in diets, which subsequently improved pig growth performance. The increased growth performance of pigs fed diets containing 0.29% or 0.35% phytate-bound P compared with pigs fed diets with 0.23% phytate-bound P is likely due to the greater concentrations of apparent total tract digestible P that was liberated from phytate in diets containing 0.29% or 0.35% phytate-bound P compared with diets with 0.23% phytate-bound P. Phytate may form complexes with metal cations and protein (Dersjant-Li and Kwakernaak, 2019), and inclusion of the PhyG in diets likely reduced the formation of insoluble phytate complexes in the gastrointestinal tract (Selle and Ravindran, 2008; Walk et al., 2013). This resulted in increased concentrations of apparent total tract digestible P, bone ash, bone Ca, and bone P (g per femur), which subsequently increased growth performance in pigs fed diets containing 0.29% or 0.35% phytate-bound P compared with diets containing 0.23% phytate-bound P.
The observation that diets containing higher levels of phytate had reduced ATTD of Ca, P, Mg, and K indicates that a significant amount of these minerals were bound to phytate (Adeola and Cowieson, 2011), which increased the proportion of unavailable minerals in diets with higher levels of phytate. At pH 5.5 and above, phytic acid occurs mainly as a complex with metal cations such as Ca2+, Mg2+, Cu2+, Zn2+, K+, and Mn2+ (Humer et al., 2015). Therefore, the formation of these complexes reduces the solubility and digestibility of minerals in the gastrointestinal tract of pigs.
In the present experiment, all basal diets were formulated to contain 0.17% standardized total tract digestible P and 0.60% total Ca. This resulted in a total Ca:standardized total tract digestible P of 3.53, which is greater than the recommended ratio (i.e., 2.15; NRC, 2012). However, it was assumed that phytase increased P digestibility along with release of Ca, and by taking the expected release values into account, the total Ca:digestible P ratio in all diets was 2.15:1. The observed increase in the ATTD of Ca and P upon phytase inclusion in the diets is in agreement with results from numerous experiments (Adeola et al., 2004; Almeida and Stein, 2012; Velayudhan et al., 2015; She et al., 2018; Arredondo et al., 2019). Both Ca and P are bound to phytate (Zeng et al., 2014), and the observed improvement in Ca and P digestibility in phytase-containing diets indicates that the bacterial 6-phytase variant hydrolyzed the Ca-phytate complexes and the ester bonds between P and the inositol ring of phytate (Adeola et al., 1995; Selle et al., 2009). Phytate may increase endogenous Na secretion in the intestinal tract of birds (Cowieson et al., 2004) and pigs (Woyengo et al., 2009), which indicates that phytate also has the ability to interact with monovalent cations (She et al., 2018). However, phytase can degrade phytate and subsequently increase Na digestibility (Cowieson et al., 2004), which was demonstrated in the present experiment. The increased digestibility of Na, K, and Mg upon phytase inclusion in the diets agrees with previous data (Kies et al., 2006; Zeng et al., 2014; She et al., 2018; Arredondo et al., 2019). The greater increase in the ATTD of P in the 0.35% phytate-bound P diet containing PhyG compared with the 0.35% phytate-bound P diet containing PhyB indicates that PhyG was more effective in liberating P bound to phytate. The PhyG was produced via fermentation of a fungal production strain that expresses a consensus bacterial 6-phytase variant gene, which has high activity in a wider pH range than PhyB (Dersjant-Li et al., 2020). The novel PhyG has high activity at very low pH (i.e., pH 1.5 to 2.0), which indicates that PhyG may quickly hydrolyze phytate and subsequently reduce the negative effect of phytate on nutrient digestibility (Christensen et al., 2020).
Calcium and P are the most abundant minerals in the animal body and are involved in many biochemical reactions and physiological functions (González-Vega and Stein, 2014). Therefore, sufficient concentrations of both Ca and P in diets are needed for bone mineralization to occur (Crenshaw, 2001). The observation that PhyG increased bone ash, bone Ca, and bone P is in agreement with data for other phytases (Braña et al., 2006; She et al., 2017; Grela et al., 2020). This observation indicates that phytase increased Ca and P utilization by increasing the ATTD of Ca and P in pigs. The observed greater response to phytase in bone ash, bone Ca, and bone P (g per femur) of pigs fed diets with higher levels of phytate compared with lower levels of phytate is likely because more apparent total tract digestible P was liberated from phytate in diets containing 0.35% or 0.29% phytate-bound P compared with diets with 0.23% phytate-bound P. However, if calculated on a percentage basis, phytase released a greater proportion of the phytate bound P in the diet with 0.23% phytate-bound P compared with diets with more phytate-bound P. The observed greater response in bone Ca and bone P of pigs fed diets containing PhyG at 1,000 FTU/kg than that of pigs fed the PhyB diets is in agreement with the observed response in P digestibility; therefore, greater P was available for bone mineralization in pigs fed the PhyG.
Conclusions
Inclusion of PhyG in diets increased the ATTD of minerals. The beneficial effect of PhyG on concentration of apparent total tract digestible P in the diets, on pig growth performance, as well as on Ca and P concentrations in bone ash of pigs was more pronounced in diets with higher phytate levels compared with low-phytate diets. This is likely due to increased hydrolysis of phosphate groups from phytate in diets with high phytate levels that increased digestibility of nutrients, which resulted in improved growth performance of pigs. The novel consensus phytase also increased ATTD of P and bone ash of pigs more than the PhyB, which may be a result of its high activity over a wide pH range.
Acknowledgment
Funding for this research by Danisco Animal Nutrition (IFF) is greatly appreciated.
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- AEE
acid-hydrolyzed ether extract
- ATTD
apparent total tract digestibility
- FTU
phytase units
- G:F
gain to feed ratio
- PhyB
Buttiauxella phytase
- PhyG
novel consensus bacterial 6-phytase variant
Conflict of interest statement
D.E.V. and Y.D.-L. are employees of Danisco Animal Nutrition (IFF), a global supplier of microbial phytase. C.D.E., M.S.F.O., and H.H.S. have no real or perceived conflicts of interest.
Literature Cited
- Adeola, O. 2001. Digestion and balance techniques in pigs. In: Lewis, A. J., and Southern L. L., editors. Swine nutrition. 2nd ed. Washington (DC): CRC Press; p. 903–916. [Google Scholar]
- Adeola, O., and Cowieson A. J.. . 2011. Board-Invited Review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi: 10.2527/jas.2010-3715 [DOI] [PubMed] [Google Scholar]
- Adeola, O., Lawrence B. V., Sutton A. L., and Cline T. R.. . 1995. Phytase-induced changes in mineral utilization in zinc-supplemented diets for pigs. J. Anim. Sci. 73:3384–3391. doi: 10.2527/1995.73113384x [DOI] [PubMed] [Google Scholar]
- Adeola, O., Sands J. S., Simmins P. H., and Schulze H.. . 2004. The efficacy of an Escherichia coli-derived phytase preparation. J. Anim. Sci. 82:2657–2666. doi: 10.2527/2004.8292657x [DOI] [PubMed] [Google Scholar]
- Almeida, F. N., and Stein H. H.. . 2012. Effects of graded levels of microbial phytase on the standardized total tract digestibility of phosphorus in corn and corn coproducts fed to pigs. J. Anim. Sci. 90:1262–1269. doi: 10.2527/jas.2011-4144 [DOI] [PubMed] [Google Scholar]
- AOAC Int. 2007. Official methods of analysis of AOAC int. 18th ed. Rev. 2nd ed. Gaithersburg (MD): AOAC Int. [Google Scholar]
- Arredondo, M. A., Casas G. A., and Stein H. H.. . 2019. Increasing levels of microbial phytase increases the digestibility of energy and minerals in diets fed to pigs. Anim. Feed Sci. Technol. 248:27–36. doi: 10.1016/j.anifeedsci.2019.01.001 [DOI] [Google Scholar]
- Bedford, M. R., and Schulze H.. . 1998. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 11:91–114. doi: 10.1079/NRR19980007 [DOI] [PubMed] [Google Scholar]
- Braña, D. V., Ellis M., Castañeda E. O., Sands J. S., and Baker D. H.. . 2006. Effect of a novel phytase on growth performance, bone ash, and mineral digestibility in nursery and grower-finisher pigs. J. Anim. Sci. 84:1839–1849. doi: 10.2527/jas.2005-565 [DOI] [PubMed] [Google Scholar]
- Christensen, T., Dersjant-Li Y., Sewalt V., Mejldal R., Haaning S., Pricelius S., Nikolaev I., Sorg R., and de Kreij A.. . 2020. In vitro characterization of a novel consensus bacterial 6-phytase and one of its variants. Curr. Biochem. Eng. 6:156–171. doi: 10.2174/2212711906999201020201710 [DOI] [Google Scholar]
- Cowieson, A. J., Acamovic T., and Bedford M. R.. . 2004. The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from broiler chickens. Br. Poult. Sci. 45:101–108. doi: 10.1080/00071660410001668923 [DOI] [PubMed] [Google Scholar]
- Crenshaw, T. D. 2001. Calcium, phosphorus, vitamin D, and vitamin K in swine nutrition. In: Lewis, A. J., and Southern L. L., editors. Swine nutrition. 2nd ed. Boca Raton (FL): CRC Press; p. 187–212. [Google Scholar]
- Dersjant-Li, Y., and Kwakernaak C.. . 2019. Comparative effects of two phytases versus increasing the inorganic phosphorus content of the diet, on nutrient and amino acid digestibility in broilers. Anim. Feed Sci. Technol. 253:166–180. doi: 10.1016/j.anifeedsci.2019.05.018 [DOI] [Google Scholar]
- Dersjant-Li, Y., Villca B., Sewalt V., de Kreij A., Marchal L., Velayudhan D. E., Sorg R. A., Christensen T., Mejldal R., Nikolaev I., . et al. 2020. Functionality of a next generation biosynthetic bacterial 6-phytase in enhancing phosphorus availability to weaned piglets fed a corn-soybean meal-based diet without added inorganic phosphate. Anim. Nutr. 6:24–30. doi: 10.1016/j.aninu.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, R. S., Lopez S., Moreira J. A., Schulin-Zeuthen M., Vitti D. M. S. S., Kebreab E., and France J.. . 2010. Application of a kinetic model to describe phosphorus metabolism in pigs fed a diet with a microbial phytase. J. Agric. Sci. 148:277–286. doi: 10.1017/S0021859610000195 [DOI] [Google Scholar]
- Ellis, R., Morris E. R., and Philpot C.. . 1977. Quantitative determination of phytate in the presence of high inorganic phosphate. Anal. Biochem. 77:536–539. doi: 10.1016/0003-2697(77)90269-X [DOI] [PubMed] [Google Scholar]
- Gizzi, G., Thyregod P., von Holst C., Bertin G., Vogel K., Faurschou-Isaksen M., Betz R., Murphy R., and Andersen B. B.. . 2008. Determination of phytase activity in feed: interlaboratory study. J. AOAC Int. 91:259–267. doi: 10.1093/jaoac/91.2.259 [DOI] [PubMed] [Google Scholar]
- González-Vega, J. C., and Stein H. H.. . 2014. Invited Review—Calcium digestibility and metabolism in pigs. Asian-Australas. J. Anim. Sci. 27:1–9. doi: 10.5713/ajas.2014.r.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grela, E. R., Muszyński S., Czech A., Donaldson J., Stanisławski P., Kapica M., Brezvyn O., Muzyka V., Kotsyumbas I., and Tomaszewska E.. . 2020. Influence of phytase supplementation at increasing doses from 0 to 1500 FTU/kg on growth performance, nutrient digestibility, and bone status in grower–finisher pigs fed phosphorus-deficient diets. Animals 10:847. doi: 10.3390/ani10050847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, C. L., Boyd R. D., Koehler D., Gould S. A., Li Q., and Patience J. F.. . 2018. The impact of “super-dosing” phytase in pig diets on growth performance during the nursery and grow-out periods. Transl. Anim. Sci. 3:419–428. doi: 10.1093/tas/txy148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humer, E., Schwarz C., and Schedle K.. . 2015. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. (Berl). 99:605–625. doi: 10.1111/jpn.12258 [DOI] [PubMed] [Google Scholar]
- Jongbloed, A. W., Mroz Z., and Kemme P. A.. . 1992. The effect of supplementary Aspergillus niger phytase in diets for pigs on concentration and apparent digestibility of dry matter, total phosphorus, and phytic acid in different sections of the alimentary tract. J. Anim. Sci. 70:1159–1168. doi: 10.2527/1992.7041159x [DOI] [PubMed] [Google Scholar]
- Kies, A. K., Kemme P. A., Sebek L. B., van Diepen J. T., and Jongbloed A. W.. . 2006. Effect of graded doses and a high dose of microbial phytase on the digestibility of various minerals in weaner pigs. J. Anim. Sci. 84:1169–1175. doi: 10.2527/2006.8451169x [DOI] [PubMed] [Google Scholar]
- Leske, K. L., and Coon C. N.. . 1999. A bioassay to determine the effect of phytase on phytate phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens. Poult. Sci. 78:1151–1157. doi: 10.1093/ps/78.8.1151 [DOI] [PubMed] [Google Scholar]
- Liao, S. F., Kies A. K., Sauer W. C., Zhang Y. C., Cervantes M., and He J. M.. . 2005. Effect of phytase supplementation to a low- and a high-phytate diet for growing pigs on the digestibilities of crude protein, amino acids, and energy. J. Anim. Sci. 83:2130–2136. doi: 10.2527/2005.8392130x [DOI] [PubMed] [Google Scholar]
- Liu, Y., Oliveira M. S. F., and Stein H. H.. . 2018. Canola meal produced from high-protein or conventional varieties of canola seeds may substitute soybean meal in diets for gestating and lactating sows without compromising sow or litter productivity. J. Anim. Sci. 96:5179–5187. doi: 10.1093/jas/sky356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. . 2004. Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington (D.C.): National Academies Press. [Google Scholar]
- Pallauf, J., Rimbach G., Pippig S., Schindler B., Höhler D., and Most E.. . 1994. Dietary effect of phytogenic phytase and an addition of microbial phytase to a diet based on field beans, wheat, peas and barley on the utilization of phosphorus, calcium, magnesium, zinc and protein in piglets. Z. Ernahrungswiss. 33:128–135. doi: 10.1007/BF01622225 [DOI] [PubMed] [Google Scholar]
- Raboy, V. 2003. myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 64:1033–1043. doi: 10.1016/s0031-9422(03)00446-1 [DOI] [PubMed] [Google Scholar]
- Selle, P. H., Cowieson A. J., and Ravindran V.. . 2009. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 124:126–141. doi: 10.1016/j.livsci.2009.01.006 [DOI] [Google Scholar]
- Selle, P. H., and Ravindran V.. . 2008. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 113:99–122. doi: 10.1016/j.livsci.2007.05.014 [DOI] [Google Scholar]
- She, Y., Liu Y., González-Vega J. C., and Stein H. H.. . 2017. Effects of graded levels of an Escherichia coli phytase on growth performance, apparent total tract digestibility of phosphorus, and on bone parameters of weanling pigs fed phosphorus-deficient corn-soybean meal based diets. Anim. Feed Sci. Technol. 232:102–109. doi: 10.1016/j.anifeedsci.2017.08.005 [DOI] [Google Scholar]
- She, Y., Sparks J. C., and Stein H. H.. . 2018. Effects of increasing concentrations of an Escherichia coli phytase on the apparent ileal digestibility of amino acids and the apparent total tract digestibility of energy and nutrients in corn-soybean meal diets fed to growing pigs. J. Anim. Sci. 96:2804–2816. doi: 10.1093/jas/sky152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, G., and Sauvant D.. . 2004. Chemical data and nutritional value. In: Sauvant, D., Perez J. M., and Tran G., editors. Tables of composition and nutritional value of feed materials. 2nd ed. Wageningen (The Netherlands):Wageningen Academic Publishers; p. 17–24. [Google Scholar]
- Velayudhan, D. E., Heo J. M., Dersjant-Li Y., Owusu-Asiedu A., and Nyachoti C. M.. . 2015. Efficacy of novel 6-phytase from Buttiauxella sp. on ileal and total tract nutrient digestibility in growing pigs fed a corn-soy based diet. Anim. Feed Sci. Technol. 210:217–224. doi: 10.1016/j.anifeedsci.2015.10.005 [DOI] [Google Scholar]
- Walk, C. L., Bedford M. R., Santos T. S., Paiva D., Bradley J. R., Wladecki H., Honaker C., and McElroy A. P.. . 2013. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 92:719–725. doi: 10.3382/ps.2012-02727 [DOI] [PubMed] [Google Scholar]
- Woyengo, T. A., Cowieson A. J., Adeola O., and Nyachoti C. M.. . 2009. Ileal digestibility and endogenous flow of minerals and amino acids: responses to dietary phytic acid in piglets. Br. J. Nutr. 102:428–433. doi: 10.1017/S0007114508184719 [DOI] [PubMed] [Google Scholar]
- Zeng, Z. K., Wang D., Piao X. S., Li P. F., Zhang H. Y., Shi C. X., and Yu S. K.. . 2014. Effects of adding super dose phytase to the phosphorus-deficient diets of young pigs on growth performance, bone quality, minerals and amino acids digestibilities. Asian-Australas. J. Anim. Sci. 27:237–246. doi: 10.5713/ajas.2013.13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, B., Lantzsch H. J., Mosenthin R., Schöner F. J., Biesalski H. K., and Drochner W.. . 2002. Comparative evaluation of the efficacy of cereal and microbial phytases in growing pigs fed diets with marginal phosphorus supply. J. Sci. Food Agric. 82:1298–1304. doi: 10.1002/jsfa.1190 [DOI] [Google Scholar]