Fig. 1. Topology of the Krebs cycle (dark blue) as precursed (green) by pyruvate 2 with glyoxylate shunt (orange) and succinate semialdehyde bypass (cyan).
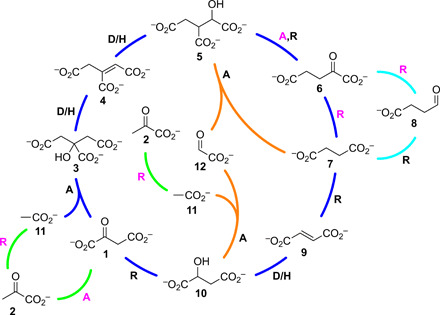
For the sake of simplicity, thioester derivatives of certain core metabolites are not shown. In the Krebs cycle, citrate 3 is converted to aconitate 4 and thence isocitrate 5 by dehydration-hydration, and the latter compound then undergoes oxidation and decarboxylation to α-ketoglutarate 6. Decarboxylation and oxidation of α-ketoglutarate 6 to succinate 7 can occur either directly or indirectly, via succinate semialdehyde 8, in a variant of the cycle (37). Dehydrogenation of succinate 7 then generates fumarate 9, which is hydrated to malate 10. The cycle is closed by aldol/Claisen-type reaction of acetate 11 with oxaloacetate 1, the oxidation product of malate 10. In the glyoxylate shunt (38), isocitrate 5 is cleaved by retro-aldol/Claisen-type reaction into succinate 7 and glyoxylate 12, and the latter is converted to malate 10 by aldol/Claisen-type reaction with acetate 11. In both the Krebs cycle proper and the glyoxylate shunt, the acetate 11 input is provided by oxidative decarboxylation of pyruvate 2. The cycle operates in the reductive sense by essentially reversing the steps; however, the epicycle (bottom left) renders the system autocatalytic due to the fact that 11 (cleaved from citrate as acetyl–coenzyme A) can undergo reductive carboxylation furnishing pyruvate 2, which can then reenter the Krebs cycle after carboxylation. R, redox reaction; D/H, dehydration/hydration; A, aldol/Claisen-type reaction; magenta letters denote that CO2 is involved in this step.
