Fig. 2. Possible mechanisms for oxidation of lactate 13 (and other α-hydroxy carboxylates) by HS−/UV light.
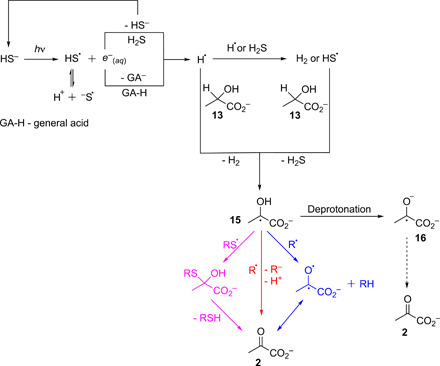
Note that reaction arrows are depicted as unidirectional for simplicity. R = H(S)n, for example. Previously, it was described in detail [see (18) , including Supplementary Discussion 2 of (18)] that H• production via irradiation of solutions of HS− at pH 6.5 is expected to be rapid alongside the production of HS•, and a simplified view is depicted in the top left. Presumably, the first step of the oxidation of 13 involves hydrogen atom abstraction from the α-position of 13, which could be performed by H• or HS•, resulting in the captodatively stabilized radical 15. Lactate radical 15 can then undergo recombination with a sulfur-centered radical (magenta pathway), with ensuing loss of the corresponding thiol/sulfide to give pyruvate 2, or hydrogen atom abstraction from the alcohol group of 15 could produce the ketone of 2 (blue pathway). Electron transfer to R• could also occur, e.g., disproportionation or reduction of a thiyl radical, which would give 2 after deprotonation of the alcohol (red pathway). Although oxidation of the ketyl radical anion 16, formed from 15, cannot be ruled out, it would seem less likely given that the pKa (where Ka is the acid dissociation constant) of the alcohol of 15 is ~9.8 (39) and the reactions were run at pH 6.5. Captodative stabilization of the transition state seems to be necessary for H• or HS• to be able to access the reaction manifold, as attempted oxidation of isopropanol under the same conditions did not yield acetone. It has been reported that H• is capable of abstracting the α-proton from α-hydroxy acids, albeit at low pH where the acids were present in protonated form (39).
