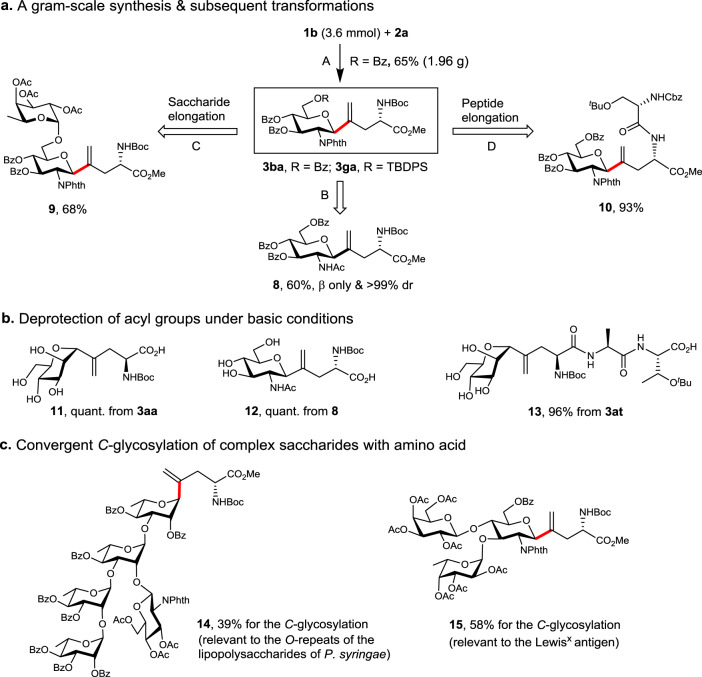
Fig. 8. Scale-up reaction, subsequent transformation, and C-glycosylation with complex saccharides.
a A gram-scale synthesis of C-glycosyl amino acid and subsequent transformations. Conditions and reagents: A. NiCl2(DME) (10 mol%), dtbbpy (15 mol%), (R)-Tol-BINAP (6.0 mol%), PMHS (2.5 equiv.), Na2CO3 (2.5 equiv.), THF (0.1 M), 25–28 °C, Ar, 48 h, 65%. B. i) 80% N2H4·H2O, MeOH, 0 °C, 9 h; ii) HOAc/MeOH (1/4, v/v), 70 °C, 1.5 h; iii) Ac2O, Et3N, CH2Cl2, 6 h, 60% over three steps. C. i) HF·pyridine, pyridine, 0 °C→rt, 2 h, 85%; ii) Au(PPh3)NTf2 (10 mol%), 4 Å MS, CH2Cl2, 0 °C→rt, 0.5 h, 81%. D. i) CH2Cl2/TFA (2/1, v/v), 0 °C→rt, 1.5 h; ii) N-Cbz-O-tBu-L-serine (1.5 equiv.), HOBt (1.5 equiv.), DIPEA (4.0 equiv.), EDCI (1.5 equiv.), DMF, -10 °C→rt, 6 h, 93% over two steps. b Deprotection of acyl groups. Conditions: LiOH (7.5 equiv), MeOH/H2O (4/1, v/v, 0.01 M), rt, 10 h. c Convergent C-glycosylation of complex oligosaccharides with amino acid, see SI for details. In red are the formed C-glycosidic bonds.