Abstract
A highly discussed step in hair sample preparation for forensic analytics is the applied decontamination. The here presented investigations aim to gain insight and give recommendations on how to conduct this decontamination for the analysis of cocaine consumption in hair. Key insights were gained from the investigation of cocaine consumer hair, which was artificially contaminated in a humid atmosphere with 13C6 labelled cocaine and from cocaine powder contaminated hair. Several decontamination protocols were investigated, whereby the usage of a decontamination protocol consisting of multiple short repetitive washes allowed to visualize the wash out of (13C6-) cocaine. Multiple methanol washes proved to be an efficient and simple decontamination approach. Our findings showed that decontamination protocols can successfully wash out recent cocaine contaminations. They were observed to be rather quickly washed out, whereas cocaine from consumption or “older” cocaine contaminations were shown to eliminate both at a constant rate (from inner hair compartments). Thus, the usage of decontamination protocols to differentiate between consumption and contamination was shown to be limited. As contamination can happen any time at any level, only the application of elaborated decision trees, based on cocaine metabolite ratios and thresholds, can provide the distinction between consumption and contamination. Thus, the authors highly recommend the usage of such tools on all hair samples analyzed for cocaine consumption.
Introduction
Forensic hair analytics is a valuable tool to track past drug exposition. Hair is especially useful for gaining retrospective insights over a prolonged time frame. For many xenobiotics, concentration changes along the hair shaft can be correlated with changes in consumption behavior over time. This is possible, as xenobiotics are incorporated via bloodstream into the non-keratinized part of the growing hair (1, 2). However, often the bloodstream is not the only incorporation pathway for a xenobiotic, which among other issues (e.g., different growth phases) complicates data interpretation. Sweat, sebum, powder and smoke may lead to external contamination followed by partial incorporation over time. To tackle this issue in particular, hair sample preparation usually includes a cleaning step using established decontamination protocols. On the one hand, these protocols aim to remove any dirt tampering with analytics, and on the other hand, they should remove those contaminating incorporations. Different decontamination protocols were published and have been discussed in the hair analytics community, but no consensus on a universal protocol has been reached so far (3–6). The present publication focuses on the suitable and proper decontamination of hair after cocaine consumption. It is well-known that cocaine consumer hair can be strongly contaminated. This can happen via sweat, where cocaine can be found in high concentrations after consumption (7) and/or via powder such as during handling cocaine and its consumption by snorting. Thus, interpretation of measured cocaine concentrations in hair is highly debated in the scientific community. A recent publication from Mantinieks et al. systematically investigated decontamination procedures and their limitations in regard to cocaine (3), whereas Tsanaclis et al. have provided a comprehensive review discussing the practical approach for interpreting the results in case external cocaine contamination is in question (6). In another recent publication, it was shown, using mass spectrometric imaging, that recent contaminations accumulate mainly in superficial hair compartments, where they are more easily washed out than cocaine incorporated into inner hair compartments (8). In this publication, also the suitability of different hair contamination models was discussed (8). It was concluded that neither the soaking model nor the powder contamination model could perfectly mimic authentic hair samples of cocaine users. Behavior of model hair and authentic hair were different using different wash protocols. The authors concluded that the best model would be somewhere in between the two models. In the present work, two new approaches were developed to gain even more insight into the cocaine contamination issue. Besides the usual models (soaked hair and powder contaminated hair), a new contamination hair model (humid atmosphere soaking) should also be evaluated for its suitability for those questions. In addition, the herein presented study includes contamination and decontamination experiments performed with 13C6-labelled cocaine. The use of 13C-labelled cocaine to spike cocaine consumer hair should help in differentiating decontamination efficiency regarding contamination and authentic cocaine incorporated via bloodstream. Soaking the hair sample in a very humid atmosphere should help in creating contaminated hair similar to authentic hair samples without continuous leaching as it can be expected during the standard soaking techniques.
The present manuscript focuses on the practical aspects of decontaminating cocaine contaminated hair. Usefulness but also limitations of very different wash protocols should be evaluated. The wash solvent of choice for the here presented experiments was methanol. Methanol was chosen as it is very well-suited for decontaminating outer as well as inner compartments of hair contaminated with cocaine (8). This is important as for proper decontamination it is not only necessary to wash off superficial contaminations but also contaminations that have been incorporated into inner compartments. As methanol is a hair swelling solvent, it is able to reach all compartments. Finally, evaluation of kinetics of washing procedures should help in differentiating contamination from intake associated incorporation. Finally, a recommendation should be given, how to differentiate possible contamination (regardless of the source) from actual intake, taking into consideration all the experimental results and existing literature on that topic.
Materials and Methods
Reagents and materials
Formic acid and ammonium formate were purchased from Sigma-Aldrich (Buchs, Switzerland). Acetonitrile and methanol (LC--MS grade) were obtained from PanReac AppliChem ITW Reagents (Darmstadt, Germany). Water was purified with a Purelab Ultra Millipore filtration unit (Labtec Services, Villmergen, Switzerland). 13C6-cocaine (labelled at the benzene ring) was purchased from Chiron (Trondheim, Norway).
Hair samples
Cocaine consumer hair and blank hair samples were provided after anonymization from the center for forensic hair analytics (Zurich, Switzerland), where these samples were examined routinely during driver’s license assessment. Cocaine and its metabolites had been quantified using a validated routine Liquid chromatography-tandem mass spectrometry (LC--MS-MS) method (see section “LC--MS-MS routine hair analysis”) analyzing the proximal 5 cm segment of the hair. The consumer hair of Experiment 1 (black, Caucasian hair) was quantified as 8,000 pg cocaine per mg hair, consumer hair of experimental Series 2 (black, Caucasian hair) as 190,000 pg/mg cocaine.
Experimental Series 1: cocaine soaked hair vs consumer hair
Soaked hair was produced according to published work from Cairns et al. by soaking 12 mg drug free hair (black, Caucasian hair) in 2 mL water containing 1 µg/mL cocaine for 1 h, followed by three times addition, mixing and removal of 2 mL water. Finally, hair was dried at room temperature (4).
Decontamination
Hair was washed without prior segmentation. For soaked hair, 10 single hairs were washed simultaneously. Due to longer hair length and limited quantities, only five consumer hairs were washed simultaneously. Cocaine soaked hair and consumer hair (8,000 pg/mg cocaine) were divided into four batches, each being washed slightly differently. Each setup contained 10 washes a 2 min in 5 mL methanol with ultra-sonication, only the last, extended wash differed: 4, 8, 12 or 16 h in 5 mL methanol.
Experimental Series 2: 13C6-cocaine contamination of consumer hair
Cocaine hair of a heavy consumer (cocaine concentration 190,000 pg/mg) was spread over absorbent paper wetted with 1mL 10 µg/mL 13C6-cocaine in water. Hair was incubated for 6 h at 37°C (Thermo Scientific Heratherm Oven, Bremen, Germany) in a parafilm® sealed box. Following this, hair was shuffled, and the absorbent paper was folded such that it covered the hair. After another 6 h of incubation, hair was once more shuffled and incubated for 1.5 h. Finally, hair was dried at room temperature.
Decontamination
Hair was washed without prior segmentation. Contaminated consumer hair was divided into three batches, each being washed differently. The following three decontamination protocols have been applied to around 9 mg of hair each.
A total of 20 times 2 min in 5 mL methanol with ultra-sonication, followed by an 18 h extended wash in 5 mL methanol.
A period of 2 min water (∼15 mL/0.1 g hair), 2 min acetone (∼10 mL/0.1 g hair), 2 min n-hexane (∼10 mL/0.1 g hair).
A period of 10 min in 5 mL methanol, three times for 3 h in 5 mL methanol.
Final amounts of cocaine in decontaminated hair
For comparison of the final amounts of cocaine in the three decontaminated hair samples (A, B or C), hair was cut into snippets, pulverized and extracted with methanol and a buffer solution (formic acid-formate) according to a published method (9, 10). After drying under a stream of nitrogen and reconstitution in the respective LC eluent, samples were measured with LC--MS-MS.
Experimental Series 3: cocaine powder contaminated hair
As described in an earlier publication, hair of two volunteers (m, Caucasian, black hair) was contaminated with cocaine by manually rubbing cocaine powder (confiscated by police on the street and analyzed for purity) on the back of their heads (for details see ref Scholz et al. (11)). Hair samples were collected 1 day and 1 week after contamination, respectively. During this time normal personal hygiene was followed. Prior to the contamination experiment, head hair had been tested negative for cocaine.
Decontamination
Collected hair strands were washed without prior segmentation using protocol A of experimental Series 2.
LC--MS-MS of methanolic wash solutions
For wash solution analysis of (13C6-) cocaine and (13C6-) benzoylecgonine, 5 µL of internal standard trimipramine-d3 was added to 500 µL of the wash solution. The mixture was vortexed, evaporated to dryness at 40°C under a nitrogen flow and reconstituted in 50 µL eluent A/B (90/10). Analysis was performed on a Thermo Fischer Ultimate 3000 UHPLC system (Thermo Fischer, San Jose, CA, USA) coupled to a Sciex 5500 QTrap linear ion trap quadrupole mass spectrometer (Sciex, Darmstadt, Germany) using a Kinetex® 1.7 µm Biphenyl 50 × 2.1 mm column. Sample injection volume was 5 µL, total run time was 4.6 min with a flow rate of 0.5 mL/min using a 10 mM ammonium formate buffer in water containing 0.1% (v/v) formic acid (pH 3.5, eluent A) and acetonitrile containing 0.1% (v/v) formic acid (eluent B). Starting conditions were 10% B hold for 0.5 min, followed by a slightly convex gradient to 90% B within 4 min, hold for 0.5 min and re-equilibrating to 10% B within 0.1 min. The Turbo V ion source equipped with a stainless steel electrode (100 μm internal diameter) was operated in positive electrospray ionization (ESI) mode with the following MS conditions: gas 1, nitrogen (50 psi); gas 2, nitrogen (60 psi); ion spray voltage, 5,500 V; ion-source temperature, 450°C; curtain gas, nitrogen (25 psi); and collision gas, nitrogen (8 psi). Analytes were measured with the following transitions and collision energies (CE): cocaine: 304.0/182.1 (CE = 25 V), 304.0/76.9 (CE = 75 V), 304.0/82.0 (CE = 35 V); 13C6-cocaine: 310.2/182.1 (CE = 25), 310.2/82 (CE = 35), 310.2/82.9 (CE = 75); benzoylecgonine: 290.0/168.1 (CE = 25), 290.0/76.9 (CE = 71), 290.0/105.0 (CE = 33); 13C6-benzoylecgonine: 296.2/168.1 (CE = 25), 296.2/111.1 (CE = 71), 296.2/82.9 (CE = 33); trimipramine-d3: 298.2/103.1 (CE = 10), 298.2/61.1 (CE = 10), 298.2/193 (CE = 10). For semi-quantitative comparison, peak areas were divided by the area of trimipramine-d3.
LC--MS-MS routine quantitative hair analysis
Quantitative analysis of hair was done according to published methods (9, 10). Briefly, hair samples (typically 3–6 cm of the proximal section) were manually washed by shaking the hairs in water, acetone and hexane for 2 min each. After drying, they were cut into snippets, pulverized and extracted with methanol and a buffer solution (formic acid-formate). After drying under a stream of nitrogen and reconstitution in the respective LC eluent, samples were quantified with LC--MS-MS.
Results and Discussion
Experimental Series 1: cocaine soaked hair and consumer hair after extended wash
Figure 1 depicts the concentration of cocaine in the wash solutions of cocaine-soaked hair washed according to the four different setups. The amount of cocaine in the wash solutions has been normalized to the second wash. This was done for semi-quantitative comparison of the four setups. The second wash was chosen for normalization, as the first wash of soaked hair usually contains a very variable amount of cocaine. In the first wash, mostly cocaine is being washed off that is loosely attached to the hair surface. For all setups, a rapid wash out of cocaine can be observed over the first 10 washes. Differences became obvious in the last extended washes of variable lengths. For the extended washes, a plateau was reached somewhere around 16 h, where no more cocaine was washed out and/or extracted. In this state, wash in and wash out of cocaine from the hair is in equilibrium with the washing solvent.
Figure 1.
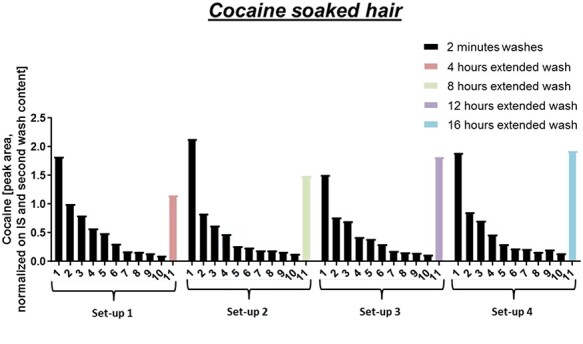
Wash solution analysis of cocaine-soaked hair for the four different setups. In each setup, hair was washed 10 times for 2 min with methanol and only the last extended wash varied in time.
Figure 2 depicts the same setting for a cocaine consumer hair (8,000 pg/mg). In typical consumer hair, cocaine has been mostly incorporated via blood stream but has also been stocked up the hair surface by sweat and sebum. Wash out of cocaine is less rapid, which can be explained by a smaller part of (contaminating) cocaine in superficial compartments compared to the soaked hair. In the first wash of the consumer hair, cocaine from sweat and/or sebum contamination is most likely washed off. After several short washes, contaminations will be washed out and analyte extraction out of inner compartments will be predominantly responsible for the detected amounts. Similar to the extended washes of the soaked hair, a plateau can be observed in the extended washes of the consumer hair around 16 h. This experiment confirms previously published findings of this group (8) that soaked (contaminating) cocaine (predominantly incorporated into superficial hair compartments) gets more rapidly washed out than cocaine incorporated into inner compartments via blood stream after consumption. It also shows that in this specific setup, washes under 4 h have not yet reached equilibrium, meaning that the amount being washed out is still bigger than the amount being washed in.
Figure 2.
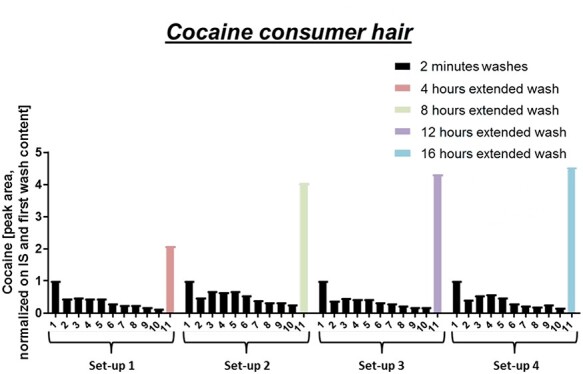
Wash solution analysis of cocaine consumer hair for the four different setups. In each setup, hair was washed 10 times for 2 min with methanol and only the last extended wash varied in time.
Experimental Series 2: 13C6-cocaine contamination of consumer hair
During this special experiment, cocaine consumer hair has additionally been contaminated with 13C6-cocaine, having the same physicochemical properties as unlabeled cocaine towards the washing steps but can be differentiated by mass spectrometry. Soaking (analyte free) hair in an analyte containing solution (as seen in the first chapter) is a common method to produce contaminated hair. This method, however, inevitably washes out some of the initially present cocaine. Thus, it would not be possible to compare the wash out of 13C6-cocaine with the wash out of unlabeled cocaine in the very same washing step. However, only by the use of (washing) solvents like water or MeOH, hair can swell, and contaminations can thus be incorporated into deeper compartments. To tackle this issue, a new approach was developed, mimicking sweat contamination. Hair was contaminated in a humid atmosphere (at 37°C) with 13C6-cocaine impregnated blotting paper (see Figure 3 for pictures of the experimental setup). By doing, so, leaching of cocaine is minimized and yet the hair can swell. For such contaminated hair, wash out of cocaine and 13C6-cocaine was investigated using three different decontamination protocols (A, B and C).
Figure 3.

Workflow for the 13C6-cocaine contamination of consumer hair in a humid atmosphere.
Wash protocol A was developed with the idea in mind of conducting several short washes with fresh solvent to speed up the washing out of contaminants. In addition, it is possible to visualize the differences in the wash out of contaminating cocaine and consumed cocaine over time. Wash solution content of (13C6-) cocaine and its metabolite and/or degradation product (13C6-) benzoylecgonine can be seen inFigure 4 for wash protocol A. The first washes showed similar amounts of cocaine and 13C6-cocaine. In the following washes, the quantities of washed out 13C6-cocaine rapidly decreased. This can be explained by a mostly superficial incorporation of 13C6-cocaine, from which it can be quickly removed. In contrast, amounts of unlabeled cocaine remained rather stable after an initial single drop. This initial drop can be explained by the easy wash out of superficial (contaminating) cocaine with the first wash. This initial drop could not be observed if hair were contaminated via typical soaking experiments. The continuous extraction of more or less equal amounts of unlabeled cocaine per step can be explained with the primary incorporation of cocaine via bloodstream into inner compartments. Benzoylecgonine and 13C6-benzoylecgonine reflect the findings for (13C6-) cocaine. Beware of different scaling for 13C6-benzoylecgonine; it was present only in minor amounts. Keep in mind that unlike unmarked benzoylecgonine, it is a product of 13C6-cocaine degradation, happening during soaking and sample preparation and not a product of active metabolism. In conclusion for wash protocol A, the rapid wash out of 13C6-cocaine indicates that final hair concentrations of unlabeled cocaine will be greater than those of 13C6-cocaine. Thus, as far as one can tell from the wash-solutions, decontamination seems to have been successful.
Figure 4.

Wash protocol A: (13C6-) cocaine (left hand side) and (13C6-) benzoylecgonine (right hand side) wash solution content in twenty 2 min wash steps and one 18 h wash step (light gray and dark gray, respectively) of cocaine consumer hair contaminated with 13C6-cocaine in a humid atmosphere. Take note, (13C6-) cocaine results are depicted on the same x-axis.
Wash protocol B was crafted according to recommendations of the Society of Hair Testing (SoHT), including washing steps with both organic solvent and aqueous solutions (9, 10). It has been in routine use since many years and has proven itself in proficiency tests. For all analytes, the initial aqueous wash of protocol B (shown in Figure 5) removed the highest amounts. On the one hand, it was the first wash, and on the other hand, water can make the hair swell. This results in an increased wash out from inner compartments. For the decreasing amounts of (13C6-) cocaine and (13C6-) benzoylecgonine in the acetone and hexane washes, not only the sequence of the washes but also solubility in the respective washing solvents can be an explanation. Once more, 13C6-cocaine was washed out at a higher rate than unlabeled cocaine, which is reflected in the higher amounts of 13C6-cocaine in all wash solvents (take note of logarithmic scale). This is not true for 13C6-benzoylecgonine, because it is exclusively formed by chemical degradation of (13C6-) cocaine. In contrast to protocol A, assessment of successful decontamination of the hair is not possible.
Figure 5.

Wash protocol B: (13C6-) cocaine (left hand side) and (13C6-) benzoylecgonine (right hand side) wash solution content in the water, acetone and hexane wash solution of cocaine consumer hair contaminated with 13C6-cocaine in a humid atmosphere. Take note of logarithmic scale.
Wash protocol C in Figure 6 is a simplified version of wash protocol A, consisting of a first short wash of 10 min in methanol to remove superficial contaminations, followed by three times 3 h washes in methanol. This protocol was tested with the idea in mind of being more practical to implement in a routine laboratory setting. As this protocol consists of multiple washes using the same solvent, wash solution analysis once more visualized how contaminating 13C6-cocaine was rather quickly washed out, whereas unlabeled cocaine remained. This quick wash out was especially visible in the first short wash, where the amount of washed out 13C6-cocaine was higher than the amount of unlabeled cocaine. In the following washes, unlabeled cocaine quantities are much higher, which can be explained with beginning extraction from inner compartments.
Figure 6.

Wash protocol C: (13C6-) cocaine (left hand side) and (13C6-) benzoylecgonine (right hand side) wash solution content in the first 10 min, and the following three times washes for 3 h in methanol of cocaine consumer hair contaminated with 13C6-cocaine in a humid atmosphere. Take note, (13C6-) cocaine results are depicted on the same x-axis.
After application of the three decontamination protocols, final amounts of labeled and unlabeled cocaine remaining in the decontaminated hair were determined. For this, hair was cut into snippets, pulverized and extracted. Amounts of remaining (13C6-) cocaine and (13C6-) benzoylecgonine in these decontaminated hair samples are given in Figure 7 for the three protocols. To compare these amounts between the different protocols, results were normalized with respect to the initial hair sample weight. Interestingly, despite of the variability in the decontamination protocols, more or less the same amounts of cocaine and benzoylecgonine were extracted. Still, differences could be found. Protocol A with multiple methanol washes proved to be the most effective decontamination protocol. This can be explained by the repetitive washes with fresh solvent each time and the extended last wash step. Protocol B showed the lowest performance, as visible from Figure 5, only the water wash removed significant amounts of cocaine. This protocol lacked multiple washes with fresh protic solvents for better performance. Protocol C, a simplified version of protocol A, showed very similar performance to A, and would therefore be eligible to replace protocol A, while reducing overall workload. Overall, only low amounts of 13C6-benzoylecgonine were extracted, showing once more that degradation of 13C6-cocaine was low (take note of different scaling). The remaining amount of 13C6-cocaine in the hair matrix after decontamination by all protocols is around 40 times lower than the amount of unlabeled cocaine. Thus, decontamination of 13C6-cocaine can be deemed to have been successful for all three protocols in this specific setup. One can assume that any other wash protocol of similar composition would be equally successful in view of such recent contamination.
Figure 7.

Semi-quantitative comparison of the (13C6-) cocaine (left hand side) and (13C6-) benzoylecgonine (right hand side) content in the hair extracts after decontamination according to protocol A, B, or C. Results were normalized with respect to the initial hair sample weight.
Experimental Series 3: cocaine powder contaminated hair
For this experiment, hair of two volunteers was contaminated with cocaine powder and collected 1 day and 1 week after contamination. Then, hair was decontaminated according to wash protocol A. For depiction of the resulting data, a new visualization was chosen. Figure 8 shows a summation of the cocaine content of the wash solutions for both volunteers at both time points. For both volunteers, hair collected 1 day after contamination shows a first-order elimination of cocaine. Wash out of cocaine from hair collected 1 week after contamination follows a zero-order elimination (linear progression). Figure 9 shows a summation of the wash solutions content of (13C6-) cocaine applying wash protocol A and C of the previous chapter. Clearly visible for both wash protocols (A and C), wash out of cocaine (in light gray) follows a zero-order elimination, whereas wash out of contaminating 13C6-cocaine (dark gray) shows a first-order elimination kinetics. Taking into account the insights from both figures, one can deduce that recent (!) cocaine contamination (regardless of the source) is washed out at a high rate, following first-order elimination. Wash out of older cocaine contaminations seems to be more problematic, because it seems to have been incorporated into inner compartments over time. Wash out of these aged contaminations followed the same elimination order as cocaine from actual consumer hair. In conclusion, determination of the washing kinetics may help in interpretation of cocaine results. Of course, this would be too much work in daily routine work in the hair laboratory. Still, these powder contaminated hair could be identified as contaminated by the usage of a decision tree recently published by our group (8, 11), additionally using cocaine metabolite ratios and thresholds.
Figure 8.

Summation of cocaine wash solution content after applying wash protocol A (18 h wash not depicted) on hair contaminated with cocaine powder. Hair from two volunteers was collected 1 day and 1 week after the contamination.
Figure 9.

Summation of (13C6-) cocaine wash solution content after applying wash protocol A (18 h wash not depicted) or wash protocol C. Cocaine consumer hair contaminated with 13C6-cocaine in a humid atmosphere was used.
Conclusion
Hair analysis for the determination of cocaine abuse is a technique with many possible pitfalls. A well-thought out sample preparation needs to be in place together with an elaborated procedure for the interpretation of the data. The here presented findings highlight the importance of using a multi wash step decontamination protocol. In doing so, a rapid wash out of recent cocaine contamination can be observed. Ideally, this would lead to a hair where cocaine incorporated from consumption will clearly be predominant in the final extract. In contrast, cocaine from actual consumption or older contaminations, which have been incorporated into inner compartments, is extracted from the hair at a constant rate. Thus, older cocaine contaminations do not differentiate from actual consumed cocaine in regard to the wash out rates. This means a decontamination protocol will in this case not be able to predominantly remove contaminating cocaine. As it is well-known, cocaine is especially prone to extreme contamination levels (e.g., as powder contamination). In the worst case, contaminant concentrations incorporated over time into inner compartments are predominant over concentrations from consumption. In such a worst-case scenario, interpretation of the concentrations will be misguided Thus, we strongly recommend to use decision trees, based on cocaine metabolite ratios and thresholds, to be able to differentiate consumption from contamination.
Acknowledgements
The authors would like to thank the staff of the Center for Forensic Hair Analytics in Zurich for their support.
Contributor Information
Robert Erne, Department of Forensic Pharmacology & Toxicology, Zurich Institute of Forensic Medicine (ZIFM), University of Zurich, Winterthurerstrasse 190/52, CH-8057, Zurich, Switzerland.
Markus R Baumgartner, Department of Forensic Pharmacology & Toxicology, Zurich Institute of Forensic Medicine (ZIFM), University of Zurich, Winterthurerstrasse 190/52, CH-8057, Zurich, Switzerland.
Thomas Kraemer, Department of Forensic Pharmacology & Toxicology, Zurich Institute of Forensic Medicine (ZIFM), University of Zurich, Winterthurerstrasse 190/52, CH-8057, Zurich, Switzerland.
Declarations of interest
The authors have no competing interests to declare.
Funding
This work was supported by the Forschungskredit of the University of Zurich [FK-18-028].
References
- 1.Pragst F., Balikova M.A. (2006) State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta, 370, 17–49. doi: 10.1016/j.cca.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 2.Cuypers E., Flanagan R.J. (2018) The interpretation of hair analysis for drugs and drug metabolites. Clinical Toxicology, 56, 90–100. doi: 10.1080/15563650.2017.1379603 [DOI] [PubMed] [Google Scholar]
- 3.Mantinieks D., Wright P., Di Rago M., Gerostamoulos D. (2019) A systematic investigation of forensic hair decontamination procedures and their limitations. Drug Testing and Analysis, 11, 1542–1555. doi: 10.1002/dta.2681 [DOI] [PubMed] [Google Scholar]
- 4.Cairns T., Hill V., Schaffer M., Thistle W. (2004) Removing and identifying drug contamination in the analysis of human hair. Forensic Science International, 145, 97–108. doi: 10.1016/j.forsciint.2004.04.024 [DOI] [PubMed] [Google Scholar]
- 5.Erne R., Bernard L., Steuer A.E., Baumgartner M.R., Kraemer T. (2019) Hair analysis: contamination versus incorporation from the circulatory system-investigations on single hair samples using time-of-flight secondary ion mass spectrometry and matrix-assisted laser desorption/ionization mass spectrometry. Analytica Chemistry, 91, 4132–4139. doi: 10.1021/acs.analchem.8b05866 [DOI] [PubMed] [Google Scholar]
- 6.Tsanaclis L., Andraus M., Wicks J. (2018) Hair analysis when external contamination is in question: A review of practical approach for the interpretation of results. Forensic Science International, 285, 105–110. doi: 10.1016/j.forsciint.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 7.Huestis M.A., Oyler J.M., Cone E.J., Wstadik A.T., Schoendorfer D., Joseph R.E. (1999) Sweat testing for cocaine, codeineand metabolites by gas chromatography–mass spectrometry. Journal of Chromatography B, 733, 247–264. doi: 10.1016/S0378-4347(99)00246-7 [DOI] [PubMed] [Google Scholar]
- 8.Erne R., Bernhard L., Kawecki M., Baumgartner M.R., Kraemer T. (2020) Using time-of-flight secondary ion mass spectrometry (ToF-SIMS) and matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS) for investigations on single hair samples to the contamination versus incorporation issue of hair analysis in the case of cocaine and methadone. Analyst, 145, 4906–4919. [DOI] [PubMed] [Google Scholar]
- 9.Rust K.Y., Baumgartner M.R., Meggiolaro N., Kraemer T. (2012) Detection and validated quantification of 21 benzodiazepines and 3 “z-drugs” in human hair by LC--MS-MS. Forensic Science International, 215, 64–72. doi: 10.1016/j.forsciint.2011.07.052 [DOI] [PubMed] [Google Scholar]
- 10.Kroll S.L., Nikolic E., Bieri F., Soyka M., Baumgartner M.R., Quednow B.B. (2018) Cognitive and socio-cognitive functioning of chronic non-medical prescription opioid users. Psychopharmacology, 235, 3451–3464. doi: 10.1007/s00213-018-5060-z [DOI] [PubMed] [Google Scholar]
- 11.Scholz C., Quednow B.B., Herdener M., Kraemer T., Baumgartner M.R. (2019) Cocaine hydroxy metabolites in hair: indicators for cocaine use versus external contamination. Journal of Analytical Toxicoly, 43, 543–552. [DOI] [PubMed] [Google Scholar]


