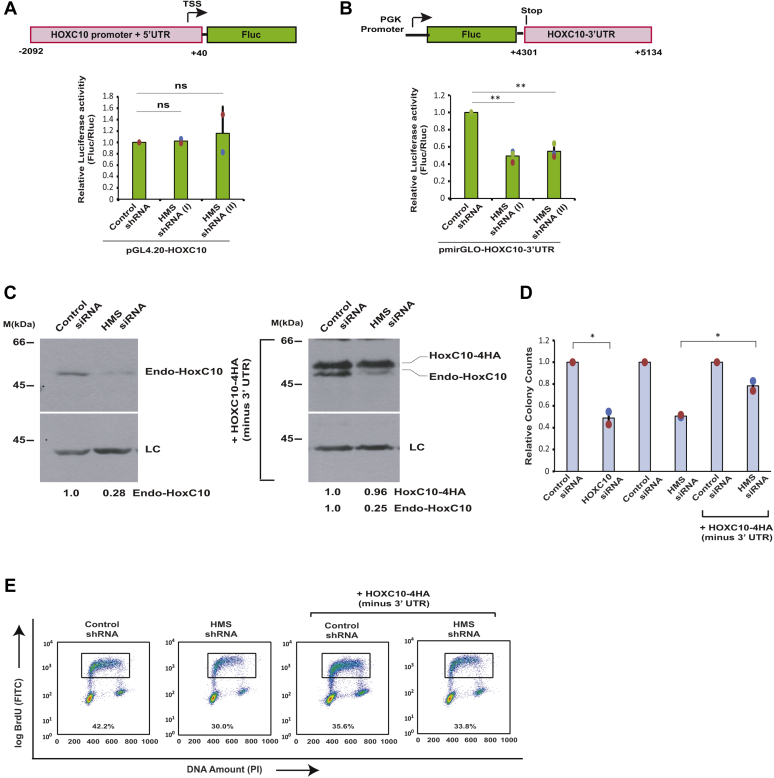
Figure 4.
HMS effect on HOXC10 occurs through the 3′UTR of HOXC10 mRNA.A, HMS depletion does not affect the HOXC10 promoter activity. Schematic representation of the reporter plasmid containing the human HOXC10 upstream region: The HOXC10 promoter and 5′UTR region spanning −2092 bp to +40 bp with respect to transcriptional start site (TSS) were used to drive expression of the firefly luciferase gene (Fluc) in promoterless pGL4.20 vector (Promega). The pGL4.20 vector containing HOXC10 promoter was transfected into control or HMS-depleted U2OS cells together with a renilla luciferase (pRL-TK) reporter vector and both luciferase activities were measured after 24 h. The relative luciferase activity in each sample is expressed as a ratio of firefly to renilla luminescence. The data represents mean of two independent experiments ±S.D. Note that both shRNA-I (ns, p = 0.899, ANOVA/Tukey’s test) and shRNA-II (ns, p = 0.830, ANOVA/Tukey’s test) do not significantly alter the luciferase activity in comparison to control shRNA samples. B, HMS depletion downregulates the HOXC10 3′UTR-fused reporter luciferase activity. An illustration of the reporter plasmid containing the human HOXC10 3′UTR region: The HOXC10 3′UTR region spanning +4301 bp to +5134 bp with respect to TSS was cloned downstream of firefly luciferase ORF under the control of PGK promoter in pmirGLO Dual-Luciferase vector (Promega). The pmirGLO vector containing HOXC10 3′UTR was transfected into control or HMS-depleted U2OS cells and 24 h later the cells were harvested and luciferase activity was measured. The relative luciferase activity in each sample was expressed as a ratio of firefly to renilla luminescence. The data represents mean of three independent experiments ±S.D. Note that both shRNA-I (∗∗p < 0.01, ANOVA/Tukey’s test) and shRNA-II (∗∗p < 0.01, ANOVA/Tukey’s test) significantly reduced the luciferase activity in comparison to control shRNA samples. C, HOXC10 expressed without the 3′UTR is impervious to HMS depletion. Stable U2OS cells expressing either a control protein (left panel) or HOXC10-4HA (without the 3′UTR) (right panel) were transfected on three consecutive days with either control GL2 or HMS siRNA. Immunoblotting with α-HOXC10 antibody demonstrates downregulation of endogenous HOXC10 in control stable cells after HMS depletion (left panel) while exogenous HOXC10-4HA remains stable after HMS depletion (right panel). D, U2OS cells stably expressing a control protein or HOXC10-4HA were transfected with either control GL2, HMS, or HOXC10 siRNA, as indicated and the cells were allowed to grow for 12 days and stained with crystal violet after which the colonies were counted. The relative colony counts are normalized to control siRNA for each treatment. The data has been represented as the mean ± SD of two independent experiments. HOXC10 depletion decreases the colony counts (∗p < 0.05, Student’s t test). Note that the colony forming ability is moderately but significantly different after HOXC10-4HA expression in HMS depleted cells (∗p < 0.05, Student’s t test). E, HOXC10 expression reverses the HMS depletion-induced S phase suppression. U2OS cells stably expressing a control or HMS shRNA were transduced with retroviral particles expressing either control protein or HOXC10-4HA, as indicated and pulsed with BrdU followed by flow cytometry to display BrdU incorporation. Individual data points have been shown for all charts.