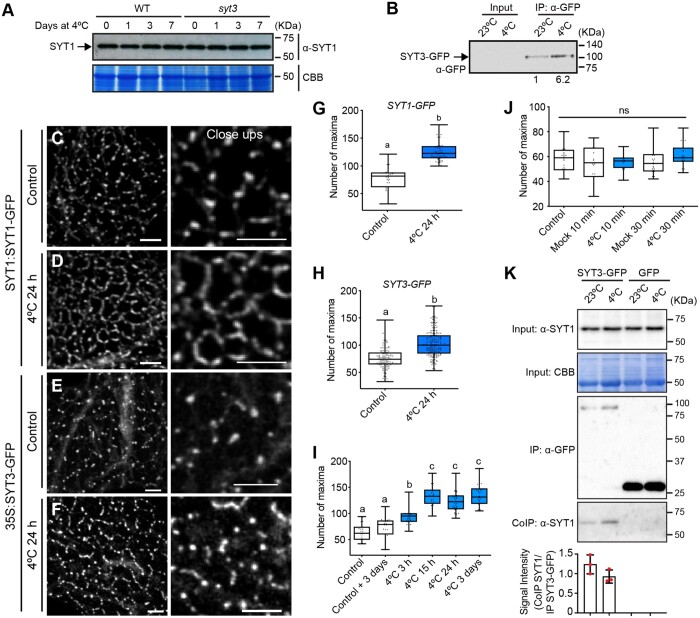
Figure 4.
SYT1 and SYT3 show cold-dependent dynamics at ER–PM CS A, Total SYT1 protein level does not change after cold treatment. Arabidopsis WT and syt3 plants were grown for 3 weeks on soil under long-day photoperiod at 20°C and then transferred to 4°C for 1, 3, or 7 d. Proteins were extracted from shoots and detected by immunoblot with anti-SYT1 antibody. Equal loading was confirmed by Coomassie blue (CBB) staining. Experiment was repeated three times with similar results. B, SYT3-GFP protein accumulation is induced by cold. Arabidopsis seedlings expressing SYT3:SYT3-GFP were grown under long-day photoperiod and 23°C for 7 d and then transferred to 4°C for 24 h or kept at 23°C. Proteins from whole seedlings were detected by immunoblot with anti-GFP antibody. No signal was detected in the crude extracts (INPUT). SYT3-GFP was concentrated using GFP-Trap beads (IP:αGFP), detected by immunoblot with anti-GFP antibody and quantified using FIJI. C–F, Representative confocal images showing SYT1-GFP (C and D) and SYT3-GFP (E and F) dynamics with cold. Arabidopsis seedlings expressing SYT1:SYT1-GFP or 35S:SYT3-GFP were grown under long-day photoperiod and 23°C for 7 d. Cold treatment plates were transferred to 4°C for 24 h (D and F), while control plates kept growing in control conditions (C and E). Mounting of the cold-treated seedlings in the coverlids was done under cold conditions and with prechilled water. Images show a maximum Z-projection of the cortical region of the epidermal cells from the cotyledon. Close ups are shown for detailed view. Scale bars 10 and 5 µm for the close-ups. G–H, Quantification of ER–PM CS labeled by SYT1-GFP (G) or SYT3-GFP (H) in cotyledons of 7-d-old Arabidopsis epidermal cells in control conditions or after 24 h at 4°C. Contacts sites were identified as intensity maxima in the cortical region using FIJI (see “Methods” for details). Dots represent individual measurements from independent ROI from at least five cotyledons. Box plots display the first and third quartiles, split by the median; whiskers extend to include the maximum and minimum values. Different lowercase letters indicate significant differences. Data were analyzed with one-way ANOVA and Tukey’s multiple comparison test; P < 0.05. I, Quantification of the amount of SYT1-GFP labeled ER–PM CS in the epidermal cells of the cotyledon of 7-d-old Arabidopsis seedlings in control conditions or after 3 h, 15 h, 24 h, and 3 d at 4°C. “Control + 3 days” is included to show that differences in the amount of CS are not due to cotyledon age. Contacts sites were identified as intensity maxima in the cortical region using FIJI (see “Methods” for details). Dots represent individual measurements from independent ROI from at least 5 cotyledons. Statistical analysis as in panels (G–H). J, Quantification of SYT1-GFP labeled contact sites in the epidermal cells of the cotyledon of 7-d-old Arabidopsis seedlings in mock conditions or after 10 and 30 min of cold treatment. Arabidopsis seedlings expressing SYT1:SYT1-GFP were grown under long-day photoperiod and 23°C for 7 d. Seedlings for cold treatment were covered with liquid half-strength MS prechilled at 4°C and the plates were transferred to a box with ice for the specified times. Mounting of the seedlings in the cover-slides was done under cold conditions and with prechilled water. Mock seedlings were covered with liquid half-strength MS at 23°C for the specified times. Contacts sites and statistical analysis as were quantified as described in panels (G–J). K, SYT1 coimmunoprecipitates with SYT3-GFP. Seven-day-old Arabidopsis WT and expressing SYT3:SYT3-GFP and 35S:GFP were subjected to 23°C and 4°C for 24 h. Total (input), IP, and CoIP proteins were analyzed by immunoblotting. Equal loading in the input was confirmed by CBB. Free GFP was used as a negative control for CoIP. SYT3-GFP and free GFP were detected with anti-GFP antibody and SYT1 was detected with anti-SYT1 antibody.