Figure 9.
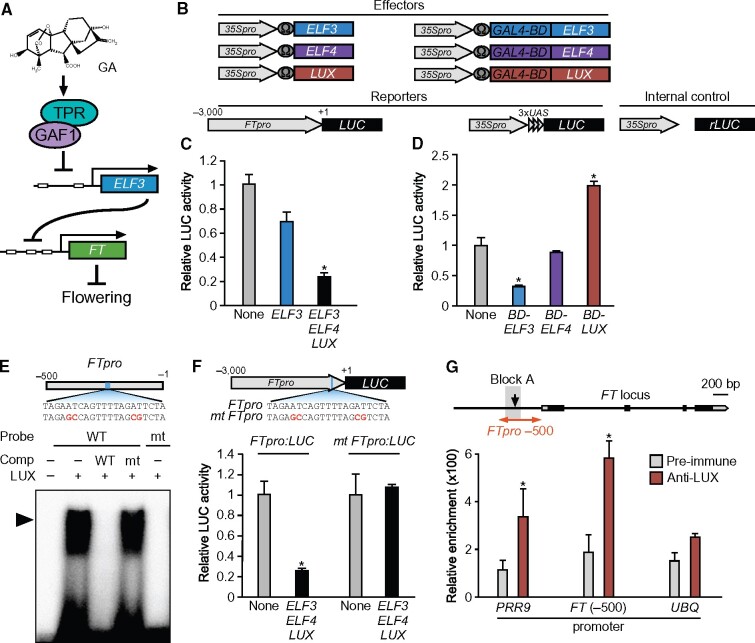
ELF3 represses the expression of FT in combination with LUX and ELF4 as part of the EC. A, GA promotes FT expression through repression of ELF3 by the GAF1-TPR complex. B, Schematic representation of the reporter and effector. A 3,000-bp fragment of the FT promoter was fused with the LUC gene. The effector plasmid expressed full-length ELF3, ELF4, LUX, GAL4BD-ELF3 (GAL4 DNA-binding domain fused ELF3), GAL4BD-ELF4, and GAL4BD-LUX under the control of the CaMV 35S promoter with a viral translation enhancer (Ω). C, Transient expression assay of ELF3, ELF4, and LUX. The effector, reporter, and internal control constructs were co-transfected into Arabidopsis protoplasts. The transfected cells were incubated for 20 h and then Luc and rLUC activities were measured. The results are shown as LUC/rLUC activity. Error bars indicate sd of three biological replicates (n = 3). Asterisks represent Student’s t test significance compared with mock treated control (*P < 0.05). D, Transient expression assay of GAL4BD-ELF3, GAL4BD-ELF4, and GAL4BD-LUX. The effector, reporter, and internal control constructs were co-transfected into Arabidopsis protoplasts. The transfected cells were incubated for 20 h and then Luc and rLUC activities were measured. The results are shown as LUC/rLUC activity. Error bars indicate sd of three biological replicates (n = 3). Asterisks represent Student’s t test significance compared with mock treated control (*P < 0.05). E, Identification of GAF1-binding regions in the FT promoter in vitro. An EMSA was performed using the recombinant LUX protein. Oligonucleotides containing FTpro (−216 to −196, wild-type; lanes 1–4) or mutated (mt) FTpro (mt; lane5) were used as probes. Red letters indicate mutated bases. Wild-type and mt indicate competition with a 1,000-fold excess of the unlabeled wild-type and mutated probe, respectively. The specific GAF1-DNA complexes are indicated by an arrowhead. +, Addition to the reaction mixtures; –, omission from the reaction mixtures. F, Transient expression assay of ELF3, ELF4, and LUX. The effector, reporter, and internal control constructs were co-transfected into Arabidopsis protoplasts. The transfected cells were incubated for 20 h and then Luc and rLUC activities were measured. The results are shown as LUC/rLUC activity. Error bars indicate sd of three biological replicates (n = 3). Asterisks represent Student’s t test significance compared with mock treated control (*P < 0.05). G, A triangle in the schematic map indicates the LUX-binding site in the FT promoter. The 200-bp region of the FT promoter used in the ChIP assay is indicated on the right. The PRR9 promoter region was used as a positive control and UBQ11 was used as a negative control. LUX binds to a region of the FT promoter in vivo. ChIP assays were performed with pre-immune serum or anti-LUX in Col-0. The level of each co-precipitated DNA fragment was quantified by real-time PCR and normalized to the input DNA. Error bars indicate sd of three technical replicates (n = 3). Asterisks represent Student’s t test significance compared with mock treated or pre-immune control (*P < 0.05). Experiments were repeated twice with independently grown plants, with similar results.