Figure 5. Commensal microbes curated from human skin restore skin barrier function via AHR activation.
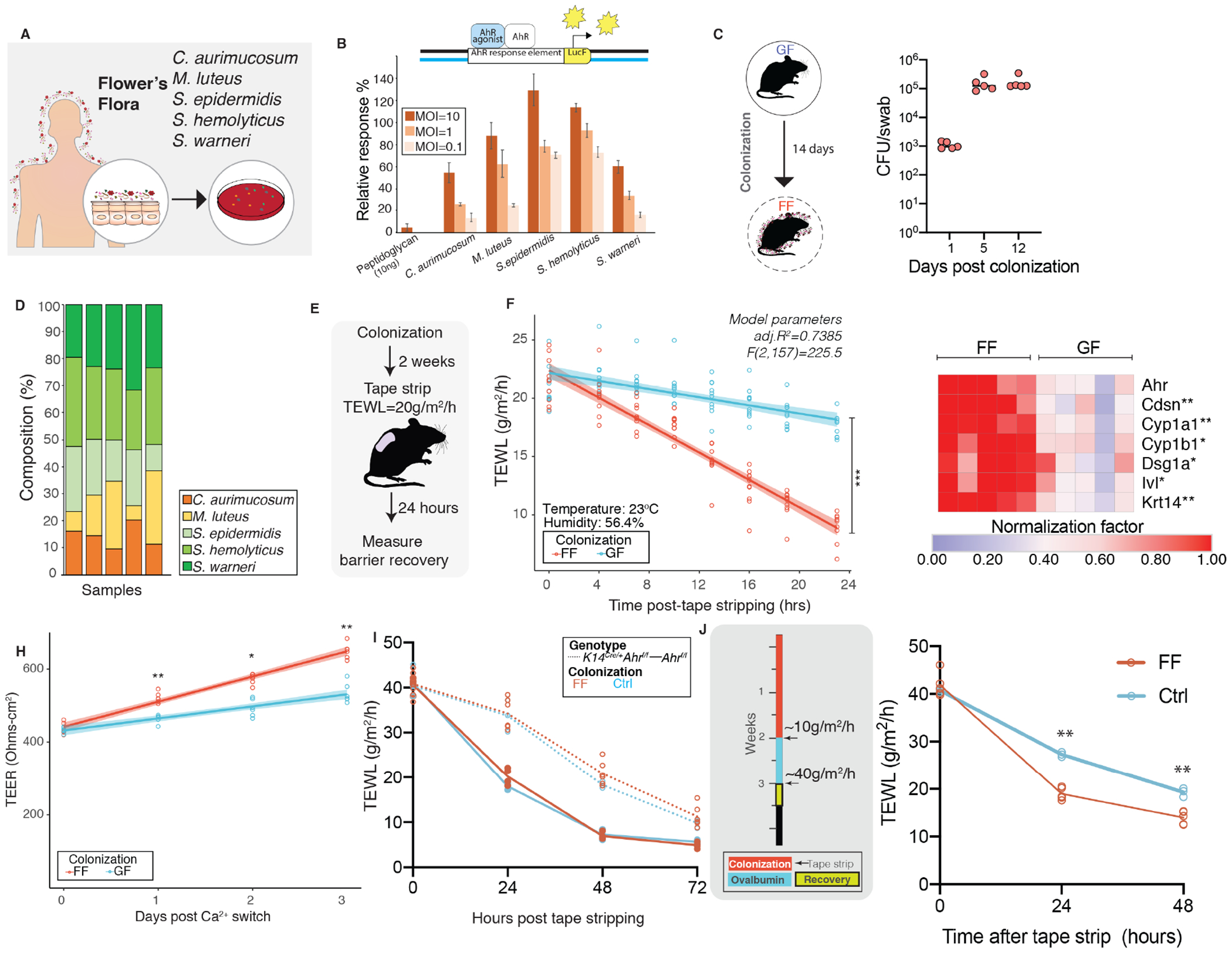
(A) Curation of bacteria for Flowers’ Flora (FF) consortium. (B) Reporter assay to assess AHR-activation in HaCaT cells using Cyp1a-luciferase reporter. Transfected cells were treated with indicated bacteria at indicated multiplicity of infection (MOI) and luminescence was measured. Relative response compared to 10nM FICZ treatment (positive control) was computed as follows: [(experimental sample ratio)-(negative control ratio)]/ [(positive control ratio)-(negative control ratio)]. (C) Germ-free mice were colonized with FF daily for two weeks. Mice were swabbed at indicated days and CFUs were enumerated. (D) To determine whether individual bacteria of FF colonized skin, qPCR analysis was conducted on genomic DNA extracted from skin swabs collected at day 14 using species-specific primers and percentage composition relative to total 16S rRNA was determined. (E) Two-weeks post colonization mice (n=5 mice/group) that were either germ-free (GF) or colonized with FF were tape stripped and (F) barrier recovery was assessed by TEWL (ANCOVA, F(1,157)=181.25, P<0.0001). (G) Expression of indicated genes was assessed by qRT-PCR in skin treated with FF or vehicle (GF). *P<0.05 and **P<0.005 by T-test, Bonferroni correction. (H) Primary mouse keratinocytes were derived, terminally differentiated and TEER was measured (**P<0.005, T-test). (I) To test if improved barrier recovery via FF is mediated through AHR, K14CreAhrf/f(n=6) were pre-colonized as shown in Fig. 4F and compared to K14CreAhrf/f (n=3) that were treated with Control (Ctrl) [ANCOVA, F(1,61)=0.1191, P=0.73115]. Additionally, Ahrf/f mice that were colonized (n=4) and untreated (n=3) were included in comparisons. (J) Experimental design to test if pre-colonization with FF could improve barrier recovery in OVA epicutaneous sensitization model. Barrier recovery kinetics were significantly improved in FF colonized versus control (non-colonized) mice [ANOVA, **P<0.01]. (See Figures S5).
