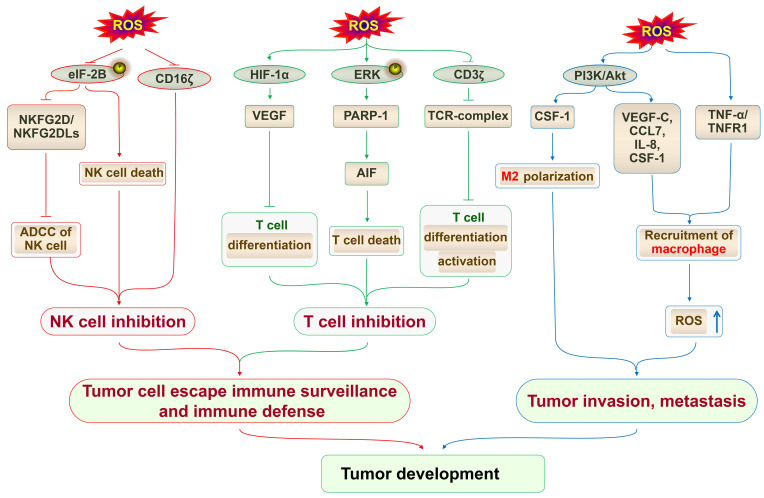
Fig 2.
The upregulation of ROS inhibits T cells and NK cells, recruits macrophages, and induces M2 macrophage polarization. The overproduction of ROS attenuates the phosphorylation of eIF2B, thereby downregulating NKG2D/NKG2Dls and suppressing NK cell-mediated ADCC activity. Elevated ROS downregulate CD16ζ expression in NK cells, thus inhibiting the cytotoxicity of NK cells. The upregulation of ROS enhances the production of VEGF via stabilizing HIF-1α, thus interfering with T-cell differentiation. ROS trigger ERK activation of PARP-1, thereby causing the release of AIF and finally leading to T-cell death. The production of ROS also decreases CD3ζ chain expression, thereby impairing the formation of TCR, and blocking the differentiation and activation of T cells. The inhibition of T cells and NK cells favors tumor development. Increased ROS activate the PI3K/Akt signaling pathway and promote the secretion of VEGF-C, CCL7, IL-8, and CSF-1, thus resulting in macrophage recruitment. ROS also activate TNF-α/TNFR1 signaling, which results in macrophage recruitment. Macrophages in turn elevate the secretion of ROS and finally promote tumor invasion and metastasis. ROS/PI3K/Akt signaling-dependent CSF-1 production induces M2 polarization of macrophages, thus contributing to tumor cell growth. NKG2DLs, NKG2D ligands; ADCC, antibody-dependent cell-mediated cytotoxicity; AIF, apoptosis-inducing factor; CCL7, cytokines chemokine (C-C motif) ligand 7.