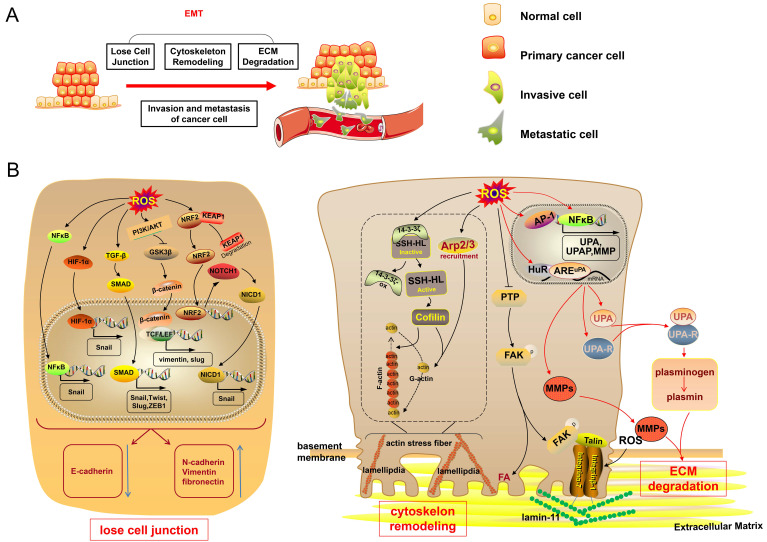
Fig 3.
Excess ROS trigger EMT, promoting cancer cell invasion and metastasis. (A) When undergoing EMT, cancer cells experience cell-cell junction dissociation, cytoskeleton remodeling and ECM degradation. These processes of EMT endow cancer cells with invasive and metastatic capacities. (B) High levels of ROS promote the expression of the transcription factors Snail, Slug, Twist and ZEB1 via the NF-κB, HIF-1α, TGF-β/SMAD, PI3K/Akt/GSK-3β and NRF2/NOTHC1/NICD1 signaling pathways, thereby repressing the expression of E-cadherin and promoting N-cadherin, Vimentin, and fibronectin, disrupting cell-cell junctions and initiating the EMT process. Elevated-ROS oxidize 14-3-3ζ and subsequently activate SSH-1L, thus resulting in the activation of cellular cofilin depolymerizing F-actin. Increased-ROS also recruit Arp2/3. Cofilin and Arp2/3, thus resulting in maintenance of actin treadmilling, formation of lamellipodia and cytoskeletal extension. Increased ROS inhibit PTP, thereby sustaining the phosphorylation of FAK, and recruiting talin, which reacts with the extracellular domain of integrin; integrin α7β1 modified by ROS links to laminin-111 in the ECM. ROS promote AP-1 and NF-κB activation of the transcription of MMPs, uPA, and uPAR; ROS also enhance the binding of HuR with AREuPA and consequently promote uPA and uPAR expression. Activated uPA leads to the transformation of plasminogen into plasmin. MMPs and plasmin result in ECM degradation. NICD1, NOTHC1 intracellular domain; N-cadherin, neural cadherin; PTP, protein tyrosine phosphatase.