Abstract
Objectives:
Prior studies have identified symptom subtypes of moderate to severe (AHI >15) obstructive sleep apnea (OSA). They have not yet been consistently examined in those with mild OSA (AHI 5–15 events/hour). This is important as women are more likely than men to present with mild OSA and may present with different OSA symptoms. The objectives of this study were to determine 1) symptom subtypes in mild OSA and 2) if there are sex differences in the distribution of subtypes.
Methods:
The sample included men (n=921) and women (n=797) with mild OSA, aged 39–90 years, evaluated with a single night of in-home polysomnography as part of the Sleep Heart Health Study. Latent class analysis determined symptom subtypes. Testing for sex differences relative to OSA severity and symptom subtype used chi-squared test for independence. Bonferroni corrected z-tests compared column proportions.
Results:
Symptom subtypes of mild OSA were not significantly different than those identified in prior studies of moderate-severe OSA (p >.05): minimally symptomatic (36.4%), disturbed sleep (11.6%), moderately sleepy (37%), and excessively sleepy (15%), p >.05. Sex differences within the symptom subtypes were significant [χ2(df = 3) = 30.04, p<.001, Cramer’s V = .132]. Relative to men, women were more likely to be in the disturbed sleep subtype (p<.05), and the excessively sleepy subtype (p<.05) while less likely to be in the moderately sleep (<.05) subtype. Women and men were equally represented in the minimal symptoms subtype (p>.05).
Conclusions:
Results suggest symptom reporting among individuals with mild OSA differs as a function of sex. These data have important clinical implications for screening men and women for OSA.
Keywords: Obstructive sleep apnea, symptom subtypes, sex differences, daytime sleepiness, disturbed sleep
1. Introduction
Obstructive sleep apnea (OSA), defined as recurrent cessation of breathing (apneas) or decreased airflow with oxygen desaturation (hypopnea) during sleep, is a serious health condition associated with adverse comorbidities and health outcomes, including mortality.1 Patients with OSA present with various symptoms including sleepiness, morning headaches, and complaints of restless sleep, among others.2 However, symptom presentation in OSA is highly heterogeneous. In efforts to understand this heterogeneity, recent studies3–6 identified that patients with OSA can be grouped into symptom subtypes, including disturbed sleep (with insomnia complaints), excessively sleepy (with severe daytime sleepiness complaints), moderately sleepy (with moderate sleepiness complaints), and minimally symptomatic (without major sleep complaints). The excessively sleepy subtype of moderate-severe OSA was the only subtype associated with increased incidence of cardiovascular diseases in the Sleep Heart Health Study.3 This indicates the clinical importance of identifying OSA symptom subtypes.
While recent studies have characterized symptom subtypes of OSA, these studies have emphasized individuals with moderate-severe OSA (apnea-hypopnea index [AHI]≥ 15).7 However, mild OSA (AHI 5–14.9) comprises a larger proportion of those with OSA than does moderate-severe OSA.8 In addition, despite the high prevalence of mild OSA, little is understood about the relevance of symptom presentation and existence of symptom subtypes in these patients. It is unclear whether symptom subtypes are similar or different in individuals with mild versus moderate to severe OSA or if symptom subtypes differ in men and women.
OSA is underdiagnosed in women. For example, the Society for Women’s Health estimates that only 1 in 10 women with OSA are diagnosed with this disorder.9 Moreover, while women are less likely to be diagnosed with OSA than men, its prevalence increases during the menopause transition.10 Several researchers have suggested that OSA in women goes unrecognized because they present with ambiguous or different symptoms of OSA than men,11–14 however, there remains little systematic research on sex differences in OSA symptom presentation. Women on average have a lower mean AHI than men; as a result, they comprise a larger proportion of the population with mild OSA (68%) than in the severe OSA category (12.2%).13 Since most studies have focused on moderate-severe OSA to examine symptom subtypes, women have been underrepresented in these studies. We hypothesize that subtypes of mild OSA can be identified from symptom presentation data, and that sex differences would be observed among subtypes at this severity level. The objectives of this study were to determine the existence of symptom subtypes in participants with mild OSA and to evaluate whether there are sex differences in the distribution of symptom subtypes. We additionally compared symptom subtypes between the mild and moderate-severe cohort.
2. Materials and Methods
2.1. Data base and sample
Data were collected from Sleep Heart Health Study (SHHS), a multi-center community-based cohort that investigated the cardiovascular consequences of sleep disordered breathing. Participants (>40yrs) completed their baseline assessments from 1995–1998. IRB approval was obtained at each investigational study site. These data are available from the National Sleep Research Resource (sleepdata.org).15,16 Symptom subtypes were assessed among 1718 participants with mild OSA (AHI 5–14.9) using thirteen symptom items as previously described in a study of moderate-severe OSA in participants from the same cohort.3 Individuals without OSA were excluded, and individuals with moderate-severe OSA were included only to understand differences in prevalence of symptom subtypes when compared against mild OSA.
Sleep electrophysiology testing was conducted by certified research technicians using Compumedics P-series portable monitor (Abbotsford, Victoria, Australia). Testing included the following channels: electroencephalogram, electrooculogram, electrocardiogram, chin electromyogram, pulse oximetry, chest and abdominal excursion by inductance plethysmography, airflow by thermal sensor, and body position. Scoring of the polysomnography is described elsewhere.17 An apnea was defined by a complete or near-complete cessation in airflow lasting for a minimum of 10 seconds and hypopneas were identified by a clear decrease in airflow or chest or abdominal plethysmograph amplitude lasting for at least 10 seconds.18 AHI was determined based on the number of apneas and hypopneas with >= 4% oxygen desaturation per hour of sleep.
Several questionnaires assessed the symptoms used to determine the subtypes (see supplement Table 1). They included the Epworth Sleepiness Scale,19 a measure of subjective excessive daytime sleepiness, Quality of Life Survey (SF 36),20 and the Sleep Habits Questionnaire18 which was developed for the parent study and captured such symptoms as snoring, waking up gasping for air, and nighttime awakenings.
2.2. Statistical Analysis
We used latent class analysis (LCA) on 14 symptom questions and the total score of the Epworth Sleepiness Scale (ESS) categorized as 0–5, 6–10, 11–15 and >15 (total of 15 symptom items). We evaluated different clusters solutions, ranging from 1–10. The best cluster solution was informed by the lowest Bayesian Information Criterion (BIC)21 and the most parsimonious scenario (e.g., lowest number of clusters), and validated by clinical interpretation of resulting clusters. Omnibus testing for sex differences in LCA assignment (i.e., symptom subtypes) were calculated using chi square test for independence. This was followed up with Bonferroni corrected z-tests to compare column proportions. Statistical analyses were conducted using R version 3.5.2 and SPSS version 25.22
3. Results
3.1. Sample Characteristics.
Sample characteristics by sex are listed in Table 1. The total sample with mild OSA included men (n=921) and women (n=797) aged 39–90 with an average age of 64.9 ± 10.9 years. The participants were primarily white (86%) and overweight (mean body mass index 28.8 ± 5.0 kg/m2), with daytime sleepiness typical of the general population (mean ESS score 7.9 ± 4.3) and a mean AHI of 9.1 ± 2.9 events/h. Women were significantly older and more overweight than men (p <.01); however, men experienced significantly more daytime sleepiness and a higher AHI than women (p <.01).
Table 1.
Sample characteristics of men and women with mild and moderate-severe obstructive sleep apnea (OSA).
| Mild OSA | Moderate-Severe OSA | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Men n=921 | Women n=797 | p | Men n=812 | Women n=395 | p | |
|
| ||||||
| Age, (mean ± SD) | 63.6 (10.8) | 66.1 (10.9) | < .001 | 65.2 (10.4) | 67.56 (10.6) | <.001 |
| Education, n (%) | < .001 | <.001 | ||||
| 0–15 years | 53.6 (457) | 72 (540) | 54.5 (416) | 39.8 (275 | ||
| >16 years | 46.4 (395) | 28 (210) | ||||
| Married, n (%) | 88.4 (796) | 67.8 (533) | < .001 | 88 (705) | 64.6 (252) | <.001 |
| Race, (n) % | .417 | .035 | ||||
| European American | 86.8 (799) | 85.2(679) | 86.5 (702) | 85.3 (337) | ||
| African American | 6.9 (64) | 8.7 (69) | 64 (7.9) | 45 (11.4%) | ||
| Other | 6.3 (58) | 6.1 (49) | 5.7 (46) | 3.3 (13) | ||
| Ethnicity, (n)% | .607 | .179 | ||||
| Not Hispanic or Latino | 96 (884) | 95.5 (761) | 95.3 (774) | 97.0 (383) | ||
| Hispanic or Latino | 4.0 (37) | 4.5 (36) | 4.7 (38) | 3.0 (12) | ||
| Hx of diabetes, (n)% | 8.8 (77) | 79 (61) | .508 | 9.6 (75) | 11.7 (45) | .270 |
| BMI, (mean ± SD) | 28.4 (4.1) | 29.2 (5.8) | < .01 | 29.9 (4.8) | 31.3 (7.1) | <.001 |
| Epworth Sleepiness Scale, (mean ± SD) | 8.4 (4.4) | 7.3 (4.1) | < .001 | 9.0 (4.7) | 7.9 (4.7) | <.001 |
| AHI, (mean ± SD) | 9.3 (3.0) | 8.8 (2.8) | < .001 | 29.1 (16.1) | 28.5 (16.8) | .555 |
Student’s t-test was conducted for continuous variables, chi-square test for independence was conducted for categorical variables; BMI= Body Mass Index [kg/m2]; AHI=Apnea Hypopnea Index
3.2. Symptom Subtypes of OSA are Present in Mild Disease.
Results of the LCA on participants with mild OSA suggested that a four-class solution was best and most parsimonious, as there was no meaningful improvement in BIC with a larger number of classes (see supplement figure 1). Importantly, the four-cluster solution was consistent with prior studies of symptom subtypes in moderate-severe OSA, therefore deemed more clinically meaningful. Figure 1 shows the conditional probabilities of the positive class of each 14 symptom items and the proportion of the ESS >10 category within each symptoms cluster. The proportions of each cluster within the total sample were as follows: disturbed sleep [11.6%], minimally symptomatic [36.4%], excessively sleepy [15.1%], and moderately sleepy [37.0%]. In addition, the mild OSA symptom subtypes in the present study were not significantly different in proportions as compared to the moderate to severe cohort (χ2(3) = .49, p >.20); [disturbed sleep (12.2%), minimally symptomatic (32.6%), excessively sleepy (16.7%), moderately sleepy (38.5%).]3 Therefore, symptom subtypes in the mild, moderate, and severe categories of OSA all displayed a similar symptom burden.
Figure 1. Spider Chart.
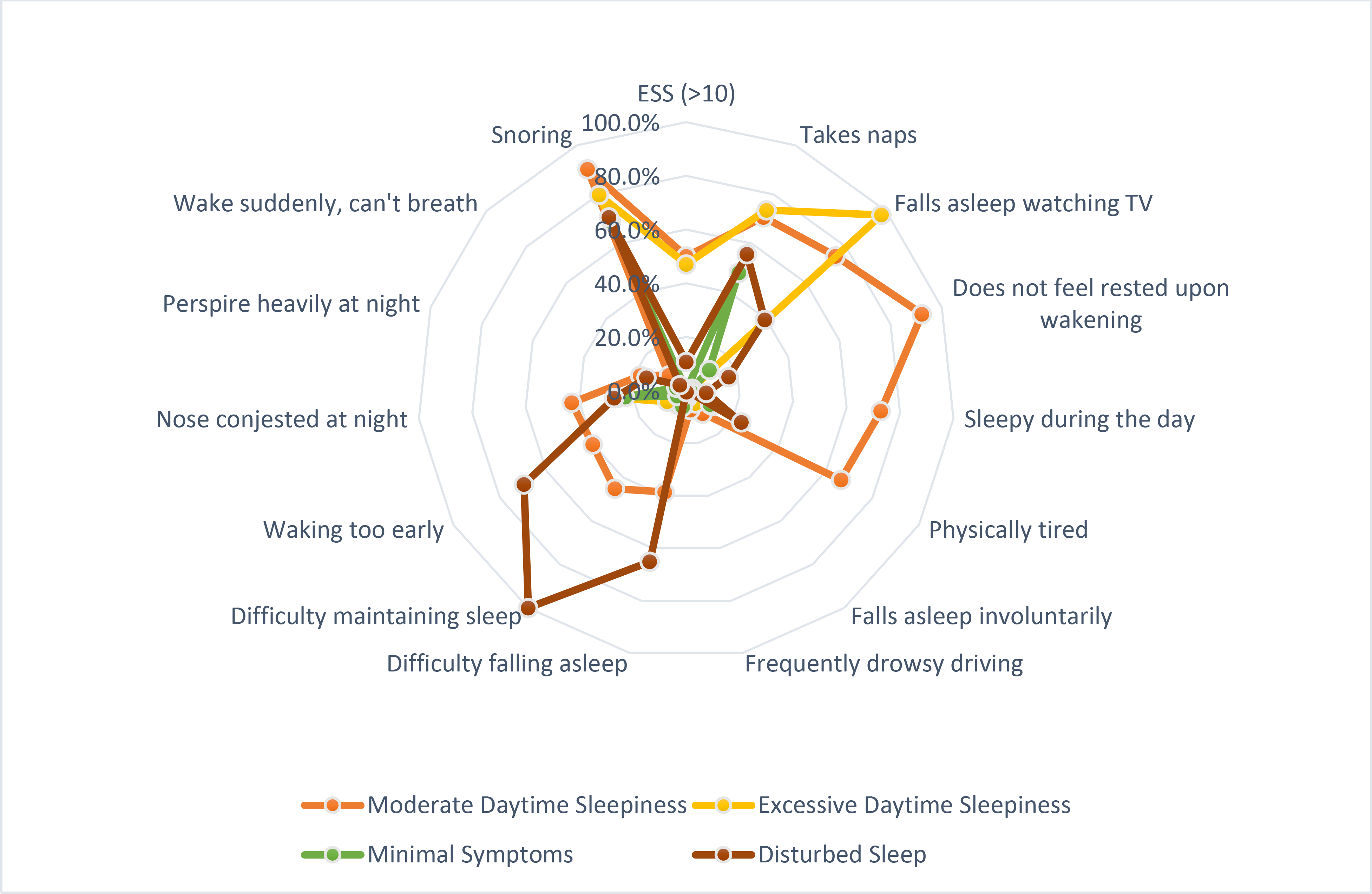
The conditional probabilities of having one of 14 symptoms within each symptoms cluster. The Epworth Sleepiness Scale was dichotomized (>10 is considered excessively sleepy).
The disturbed symptom subtype includes symptoms typical of insomnia such as difficulty falling asleep, maintaining sleep, and early rising. The moderately sleepy subtype was similar to the excessively sleepy subtype in endorsements of the ESS questions, but those in the moderately sleepy subtype were less likely to endorse the following questions “do not feel rested in the morning,” “sleepy during the day,” and “physically tired.” The “minimally symptomatic” subtype reflected few reports of daytime sleepiness or disturbed sleep.
3.3. Significant Sex Differences in Symptom Subtype Presentation in Mild OSA
Sex differences in proportions of men and women were found amongst subtypes of mild OSA (Table 2, χ2(3) = 30.04, p<0.001). Table 2 shows the total numbers of men and women within each subtype, the percentages of each subtype within each of the male and female cohorts respectively, and standardized residuals for men and women within each subtype. Women were significantly more likely to be observed in the disturbed sleep subtype than men, while men were significantly more frequently observed in the moderately sleepy subtype than women. While women were significantly more frequently observed than expected in the excessively sleepy subtype than men, this sex difference was not as pronounced as the disturbed sleep or moderately sleepy subtype. Both women and men were evenly represented in the minimal symptoms subtype.
Table 2.
Total (n) & percentage (%) of men and women in each symptom cluster and standardized residuals (SR) of symptom subtypes with mild OSA
| Minimal symptoms | Disturbed Sleep* | Moderately Sleepy* | Excessively sleepy* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| n | % | SR | n | % | SR | n | % | SR | n | % | SR | |
|
| ||||||||||||
| Men | 334 | 36.3 | −0.1 | 83 | 9 | −2.3 | 385 | 41.8 | 2.4 | 119 | 12.9 | −1.7 |
| Women | 291 | 36.5 | 0.1 | 115 | 14.6 | 2.5 | 250 | 31.4 | −2.6 | 140 | 17.6 | 1.8 |
p<.05
While the Centers for Medicaid and Medicare use the definition of AHI that includes all apneas plus hypopneas defined by a 30% drop in flow lasting at least 10 seconds and associated with a 4% oxygen desaturation (AHI-4%), the current AASM recommendation is for hypopneas defined by a 3% desaturation or an EEG arousal (AHI-3%/arousal). A posthoc analyses found significant sex differences in the 3% desaturation measures with and without arousals similar to sex differences in the 4% desaturation category.
3.4. Mild OSA vs. Moderate to Severe OSA by total sample and by sex.
Whereas women made up only32 % of SHHS participants with moderate to severe OSA, they made up 46% of the sample with mild OSA. Because the SHHS cohort with moderate to severe OSA was underpowered to examine sex differences in symptom subtypes, we present the results in Figure 2 for descriptive purposes and consideration for future research only. Men are more likely to be in the moderately sleepy subtype than women in both the mild and moderate-severe cohorts while women are more likely to be found in the disturbed sleep subtype in both the mild and moderate-severe cohort.
Figure 2. Graph.
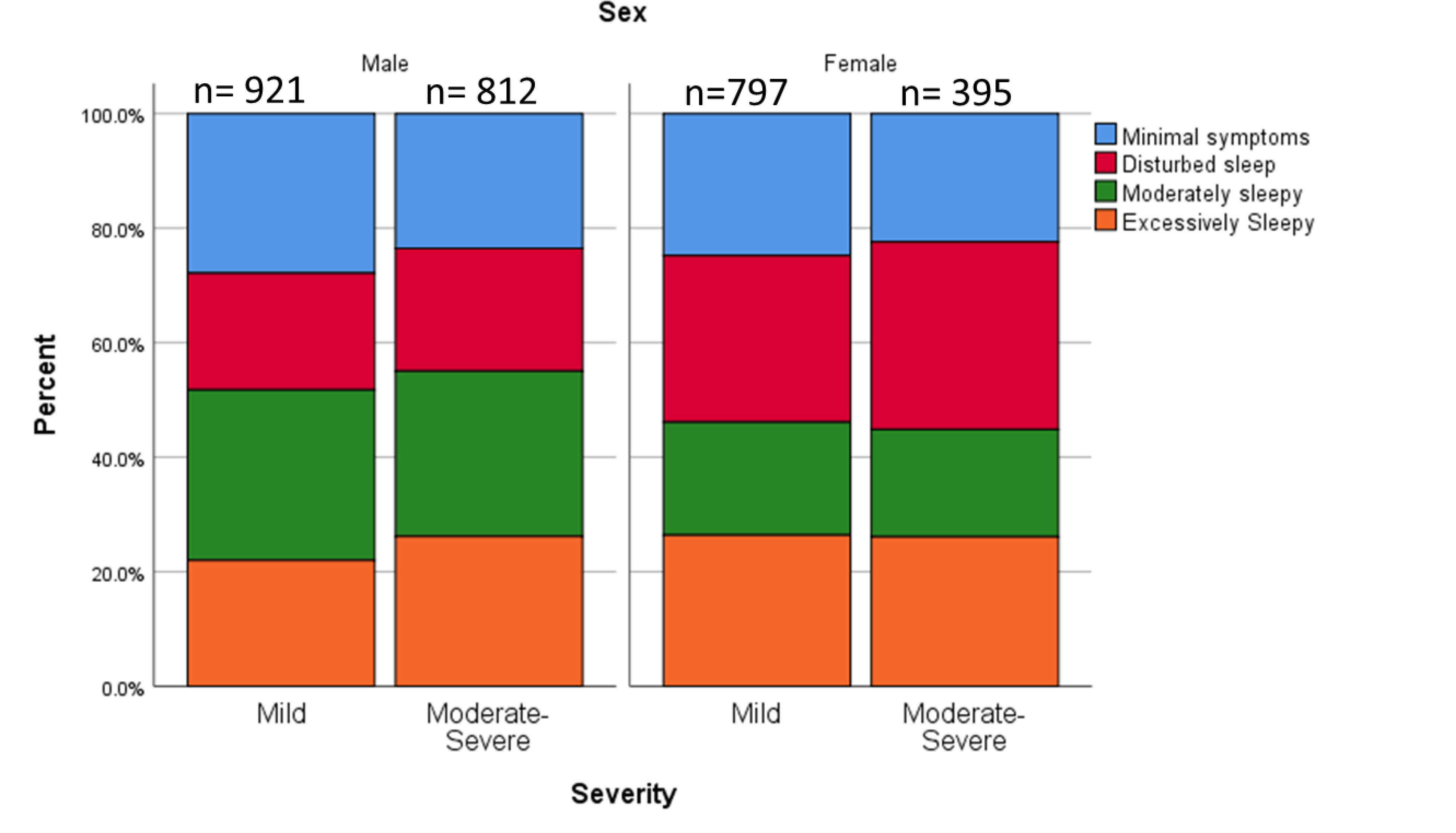
Comparison of the proportions of symptom subtypes by sex and OSA severity.
4. Discussion
In the present study we have identified symptom subtypes in adult men and women with mild OSA and evaluated sex differences in symptom subtypes. We found substantial similarities in symptom subtypes between the mild and moderate-severe categories of OSA, including a similar four-class solution of subtypes, and similar proportions among those subtypes. These findings suggest analogous symptom presentations across the spectrum of sleep-disordered breathing severity levels based on the AHI. Individuals who present with sleep apnea report similar types of symptoms and at similar proportions, regardless of OSA severity. We also found significant sex differences in symptom reporting in men and women with mild OSA. Among individuals with mild OSA, women were more likely than men to report symptoms related to disturbed sleep while men were more likely than women to present with symptoms of moderate daytime sleepiness. These significant sex differences within adults with mild OSA suggest that sex may influence either the experience, perception, or reporting of symptoms of OSA. Ramifications of not considering women’s symptoms in OSA research are that their female-specific symptom presentation may continue to go unrecognized, leading to underdiagnoses, and under-treatment, with consequently greater risk for OSA-related morbidity.
The symptom subtypes in our sample of participants with mild OSA are similar to other studies that have assessed symptom clusters in participants with moderate-severe OSA severity4,5,7. While differences were observed in previous studies regarding the number of symptom subtypes, most have found three to four symptom clusters similar to this study, usually with three major groups: “excessively sleepy”, “minimal symptoms” and “disturbed sleep”.7 Our findings suggest that despite having mild OSA, participants in the SHHS cohort still could be categorized according to their symptom profile into the same subtypes as those with moderate-severe OSA. This is evidenced by approximately two-thirds of the participants within all categories (mild, moderate, severe) experiencing excessive or moderate daytime sleepiness or disturbed sleep. At the same time, approximately one-third of the sample in all OSA categories reported minimal to no recognized symptoms of OSA. Lack of significant differences in the proportion of symptom subtypes between the mild and moderate-severe cohorts suggest that the AHI-based OSA severity categories alone do not indicate severity of symptoms. There has been debate as to whether AHI is a sufficient metric for OSA in terms of disease severity and health outcomes.3,23–25 Our findings corroborate studies that have suggested AHI does not provide a comprehensive measure of OSA severity.
Women were significantly more likely than men to express the disturbed sleep subtype in all categories of OSA severity. This finding is consistent with several retrospective studies in which more women than men reported insomnia symptoms at the time of their diagnosis of OSA.26–28 Based on these retrospective studies, it has been posited that women with OSA are more likely than men with OSA to present with insomnia.9,29 A recent study characterizing OSA presentation provided a summary of four OSA subtypes based on a literature review including not only to symptoms, but also effects of treatment and long-term outcomes.7 One phenotype, “subtype C,” was described in predominately middle-aged women with symptoms of insomnia. Consistent with prior literature, the present study finds that a significant subset of women with OSA present with symptoms of insomnia. Several mechanisms have been posited that explain the propensity for comorbid OSA and insomnia. The airway is most vulnerable to collapse at the wake-sleep transition and individuals who awaken frequently may therefore have more frequent episodes of airway collapse at this transition. It is also possible that awakenings due to OSA will be prolonged in those with a propensity towards insomnia leading to insomnia as the presenting complaint. 30,31 However, most studies that have investigated the relationships between comorbid OSA and insomnia have not reported sub analyses by sex. Therefore, we suggest more research is needed to uncover if there is a mechanistic relationship between OSA and insomnia in women, or to learn if this symptom is simply reflective of the high rate of insomnia in the general female population.
Men were significantly more likely than women to express the moderately sleepy subtype. This subtype is similar to the previously described “subtype B,” where men were described as having “minimal to moderate symptom burden.”7 When examining this symptom subtype, we see in our sample that while this group endorsed involuntary daytime sleeping and napping, they were less likely to endorse a generalized daytime burden including the following symptoms: “sleepy during the day,” “do not feel rested at awakening,” or “physically tired.” It has been suggested that persons in this group may not recognize their own symptoms possibly because these participants were older and have grown inured to their symptoms of sleepiness. In the present study, the average age of participants was in their mid-sixties, suggesting they may also have been inured to their symptoms. Systematic evaluation of interactions among OSA severity and symptoms by age may shed further light on this group, and in the overall progression of symptom presentation over the course of the disease. However, these findings may also have social rather than biological roots. Sociologists have previously posited that some men view sleep and napping simply as a pragmatic event based on a bodily ‘need’ for sleep.32 While these men may be clinically sleepy, they may not perceive it as a burden in the same sense that the participants in the excessively sleepy group do. Consequently, these findings may reflect the different ways that people perceive or report daytime sleepiness rather than a difference in intensity of daytime sleepiness. Conversely, the stronger association of the excessively sleepy versus the moderately sleepy subtype with cardiovascular disease outcomes suggests some degree of physiological difference between these OSA subtypes.3 Further studies are necessary to disentangle gendered expectations, roles and behaviors from reports of actual daytime sleepiness to ensure that both men and women are effectively evaluated. Moreover, objective sleepiness measures in addition to self-report may be relevant to distinguish the underlying biological or psychosocial basis of a patient’s sleepiness.
Differences in the proportions of men and women within the disturbed sleep and moderate sleepiness subtypes of mild OSA, as well as sex differences in the proportions of mild vs. moderate-severe OSA, have possible clinical implications for screening women and men for OSA. While the traditional risk factors and symptoms of OSA include male sex, obesity, snoring, and excessive daytime sleepiness, our findings corroborate previous research that complaints of disturbed sleep or insomnia may also have a strong relationship with OSA but more so in women, even among those with more severe OSA as determined by AHI. Clinicians who treat insomnia, particularly in middle-aged women whose risk for OSA increases as they enter the menopausal transition,10 should be sure to screen for this serious medical disorder which may contribute to and exacerbate insomnia symptoms, particularly difficulty maintaining sleep.2 Additionally, asking men about their subjective sleepiness during the day may not yield information nor be equivalent to information about their specific involuntary sleeping or voluntary napping habits.
The present study has several strengths, such as the use of a large community-based sample of older adults of all OSA severity levels, allowing accurate comparison between mild and moderate-severe cases. Future investigations on sex differences in cardiovascular risk among different OSA subtypes would be more powered than in moderate-severe patients alone33–35 On the other hand, the study has limitations that could impact generalizability of findings, as the SHHS cohort is a sample of older participants primarily of European-American descent. For this analysis, we utilized a hypopnea event definition requiring a 4% desaturation, as required by the Center for Medicare and Medicaid Studies for reimbursement of positive airway pressure therapy. Whether the current findings would apply to people with even milder severity of OSA, as might be identified utilizing hypopnea definitions requiring a 3% desaturation or arousal, is not known.
In conclusion, our findings suggest that OSA symptom subtypes are not exclusively observed in moderate-severe OSA. High symptom burden is found within every category of severity, but it may be experienced differently between men and women. These findings suggest that ongoing studies assessing OSA phenotypes should include participants of all OSA severity levels. The inclusion of mild OSA into these studies additionally ensures that women will be adequately represented in studies to determine associations of OSA and disease outcomes in general populations
Supplementary Material
Symptom subtypes in mild OSA were similar to previous studies of moderate-severe OSA: excessive daytime sleepiness, moderate daytime sleepiness, disturbed sleep, and minimal symptoms.
Relative to men, women were more likely to be in the disturbed sleep subtype, the excessively sleepy subtype, and less likely to be in the moderately sleepy subtype.
Women and men were equally represented in the minimal symptoms subtype.
High symptom burden is found within every category of OSA severity, but may be experienced differently between men and women.
Acknowledgments
Statistical consultation was provided by Paul W. Scott PhD.
Disclosure Statement
Financial Disclosure: Dr. Gottlieb reports grants from National Institutes of Health, during the conduct of the study.
Non-financial Disclosure: Dr. Gottlieb reports non-financial support from ResMed, Inc. inclusive of sleep apnea testing devices for conduct of an investigator-initiated pilot research study
Research/Grant Support: NHLBI: T32-HL-007779 (PI: D. Buysse); the American Academy of Sleep Medicine Foundation (PI: D. Mazzotti).
The Sleep Heart Health Study was supported by the NHLBI through the following cooperative agreements: U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL63463 (Case Western Reserve University), U01HL53937 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53 934 (University of Minnesota), U01HL63429 (Missouri Breaks Research), and U01HL539 31 (New York University). The National Sleep Research Resource was supported by the NHLBI (HL114473).
Footnotes
Sleep Heart Health Study Data Coordinating Center, https://clinicaltrials.gov/ct2/show/NCT00005275, NCT00005275
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenberg H, Lakticova V, Scharf SM. Obstructive Sleep Apnea. In: Principles and Practice of Sleep Medicine. Elsevier; 2017:1110–1124.e6. doi: 10.1016/B978-0-323-24288-2.00114-8 [DOI] [Google Scholar]
- 2.Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA - J Am Med Assoc. 2020;323(14):1380–1400. doi: 10.1001/jama.2020.3514 [DOI] [PubMed] [Google Scholar]
- 3.Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. doi: 10.1164/rccm.201808-1509OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan BT, Kim J, Singh B, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: A cluster analysis. Sleep. 2018;41(3). doi: 10.1093/sleep/zsx214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur Respir J. 2014;44(6):1600–1607. doi: 10.1183/09031936.00032314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Keenan BT, Lim DC, Lee SK, Pack AI, Shin C. Symptom-Based subgroups of koreans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(3):437–443. doi: 10.5664/jcsm.6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinchuk A, Yaggi HK. Phenotypic Subtypes of OSA. Chest. Published onlineSeptember2019. doi: 10.1016/j.chest.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AM, Mong J, Baker F, et al. Women and Sleep A Guide for Better Health.; 2017. Accessed January 28, 2018. https://swhr.org/wp-content/uploads/2017/11/SWHR_Women-Sleep-Guide.pdf
- 10.Mirer AG, Young T, Palta M, Benca RM, Rasmuson A, Peppard PE. Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study. Menopause. 2017;24(2):157–162. doi: 10.1097/GME.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wimms A, Woehrle H, Ketheeswaran S, Ramanan D, Armitstead J. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. Biomed Res Int. 2016;2016:1764837. doi: 10.1155/2016/1764837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41(3):610–615. doi: 10.1183/09031936.00212711 [DOI] [PubMed] [Google Scholar]
- 13.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye L, Pien GW, Ratcliffe SJ, Weaver TE. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J Clin Sleep Med. 2009;5(6):512–518. Accessed June 27, 2015. /pmc/articles/PMC2792965/?report=abstract [PMC free article] [PubMed] [Google Scholar]
- 15.Dean DA, Goldberger AL, Mueller R, et al. Scaling Up Scientific Discovery in Sleep Medicine: The National Sleep Research Resource. Sleep. 2016;39(5):1151–1164. doi: 10.5665/sleep.5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang GQ, Cui L, Mueller R, et al. The National Sleep Research Resource: Towards a sleep data commons. J Am Med Informatics Assoc. 2018;25(10):1351–1358. doi: 10.1093/jamia/ocy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang GQ, Cui L, Mueller R, et al. The National Sleep Research Resource: Towards a sleep data commons. J Am Med Informatics Assoc. 2018;25(10):1351–1358. doi: 10.1093/jamia/ocy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SF Q, BV H, C I, et al. The Sleep Heart Health Study: Design, Rationale, and Methods. Sleep. 1997;20(12). doi: 10.1093/sleep/20.12.1077 [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. Accessed October 30, 2014. http://www.ncbi.nlm.nih.gov/pubmed/1798888 [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. doi: 10.1513/pats.200708-137MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IBM Corp. IBM SPSS Statistics for Windows.
- 23.Rapoport DM. POINT: Is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? Yes. Chest. 2016;149(1):14–16. doi: 10.1378/chest.15-1319 [DOI] [PubMed] [Google Scholar]
- 24.Kapur VK, Donovan LM. Why a single index to measure sleep apnea is not enough. J Clin Sleep Med. 2019;15(5):683–684. doi: 10.5664/jcsm.7746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Punjabi NM. COUNTERPOINT: Is the Apnea-Hypopnea Index the Best Way to Quantify the Severity of Sleep-Disordered Breathing? No. Chest. 2016;149(1):16–19. doi: 10.1378/chest.14-2261 [DOI] [PubMed] [Google Scholar]
- 26.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28(3):309–314. Accessed June 27, 2015. http://www.ncbi.nlm.nih.gov/pubmed/16173651 [PubMed] [Google Scholar]
- 27.Greenberg-Dotan S, Reuveni H, Simon-Tuval T, Oksenberg A, Tarasiuk A. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep. 2007;30(9):1173–1180. Accessed June 27, 2015. /pmc/articles/PMC1978412/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian S, Guntupalli B, Murugan T, et al. Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep Breath. 2011;15(4):711–715. doi: 10.1007/s11325-010-0426-4 [DOI] [PubMed] [Google Scholar]
- 29.Mallampalli MP, Carter CL. Exploring sex and gender differences in sleep health: a Society for Women’s Health Research Report. J Womens Heal. 2014;23(7):553–562. doi: 10.1089/jwh.2014.4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björnsdóttir E, Janson C, Sigurdsson JF, et al. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–1909. doi: 10.5665/sleep.3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen HCJP, Venekamp LN, Peeters GAM, Pijpers A, Pevernagie DAA. Management of insomnia in sleep disordered breathing. Eur Respir Rev. 2019;28(153). doi: 10.1183/16000617.0080-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadows R, Arber S, Venn S, Hislop J. Engaging with sleep: Male definitions, understandings and attitudes. Sociol Heal Illn. 2008;30(5):696–710. doi: 10.1111/j.1467-9566.2008.01088.x [DOI] [PubMed] [Google Scholar]
- 33.Campos-Rodriguez F, Asensio-Cruz MI, Cordero-Guevara J, et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: A randomized controlled trial. Sleep. 2019;42(10). doi: 10.1093/sleep/zsz145 [DOI] [PubMed] [Google Scholar]
- 34.Seif F, Patel SR, Walia HK, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32(2):267–275. doi: 10.1097/HJH.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao YY, Wang R, Gleason KJ, et al. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best apnea interventions for research (BestAIR) Trial. Sleep. 2017;40(4). doi: 10.1093/sleep/zsx040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


