Figure 1. Divergent primary sex determination signals in Diptera converge on sex-specific doublesex splicing.
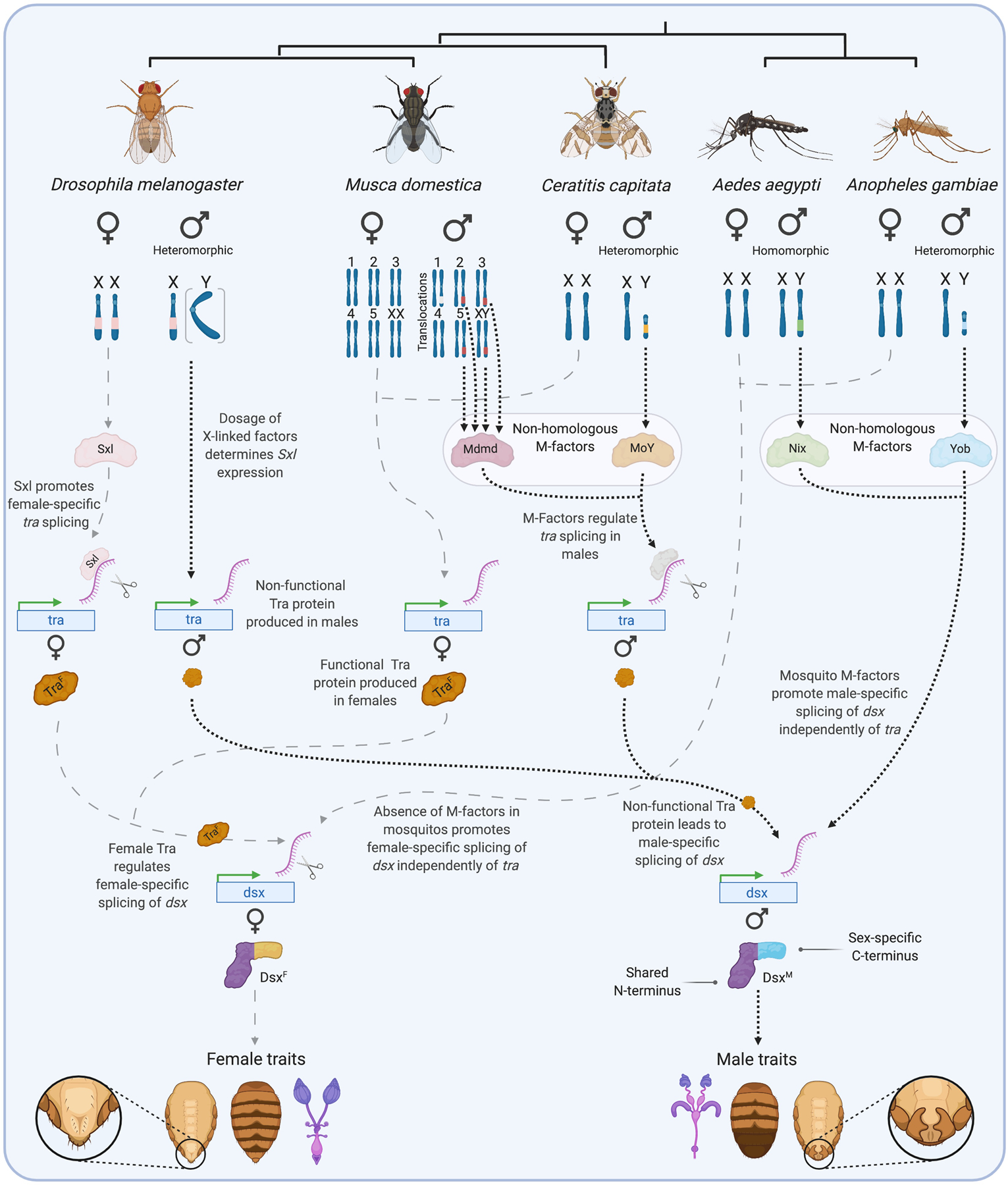
In the 5 Dipterans shown, sex is specified at the level of the individual cell by factors associated with sex (or proto-sex) chromosomes. These male- and female-defining chromosomes vary between species from being highly similar to each other (homomorphic) to highly divergent (heteromorphic) in morphology and gene content. In D. melanogaster, the number of X chromosomes determines the dosage of a set of X-linked factors that regulate the expression state of Sex lethal (Sxl). High dosage (XX) activates Sxl expression, the protein product of which promotes female-specific splicing of transformer (tra). The resulting female-specific isoform of Transformer protein (TraF) is required for the female-specific splicing of the transcription factor doublesex (dsx). Maleness is defined by the lower dosage of X-linked factors, rather than the presence of a Y-chromosome (e.g., X0 individuals are males). Having a single X chromosome leaves Sxl inactive in males, and the male-specific isoform of Transformer is produced (TraM). The presence of a premature stop codon renders TraM non-functional, which in turn leads to the production of the male-specific isoform of dsx. Musca domestica, Ceratitis capitata, Aedes aegypti, and Anopheles gambiae each use independently evolved (non-homologous) dominant M-factors to determine maleness. These are encoded on the Y-chromosome in most cases, but translocations to autosomes (turning them into proto-sex chromosomes) have been detected in different M. domestica populations. Whether the M-factor found on chromosome 1 in one population of M. domestica (shown in white) is a derived Mdmd sequence or an independently evolved M-factor remains unclear. In M. domestica and C. capitata, the presence of M-factors leads to the production of non-functional TraM and therefore, as in D. melanogaster, the production of the male-specific isoform of Dsx. No tra homolog has been found in Ae. aegypti or An. gambiae. Their M-factors, Nix and Yob respectively, are therefore presumed to determine the male-specific splicing of dsx by an as of yet unknown, tra-independent mechanism. The male and female isoforms of Dsx share a DNA-binding N-terminus but bear different C-termini, allowing them to regulate downstream target genes in a sex-specific manner, leading to the development of sex-specific traits. Figure created using BioRender.
