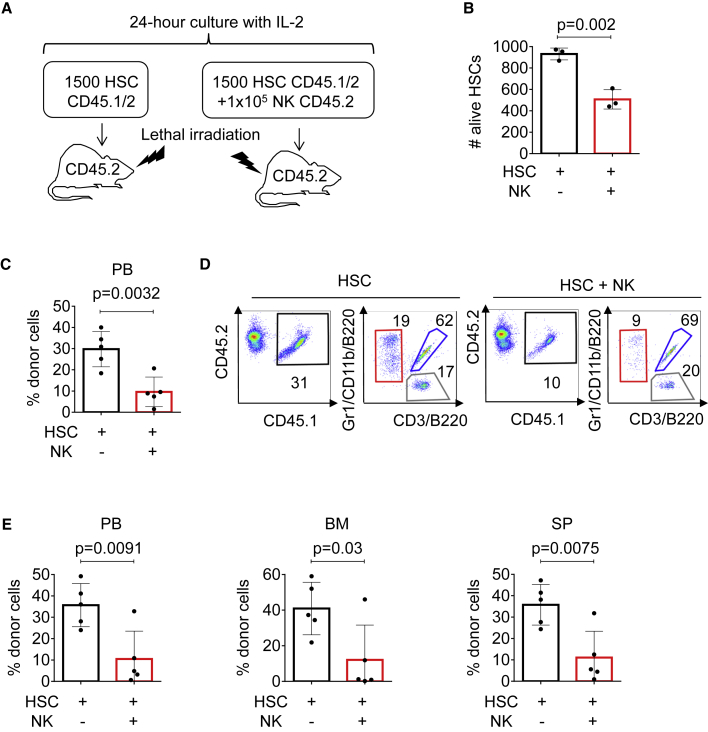
Figure 2.
NK cell exposure compromises HSC engraftment in vivo
(A) Experimental strategy for NK and HSCT assays. HSCs from C57BL/6 CD45.1/2 mice were purified by sorting, cultured in the presence or absence of NK cells (Ter119− CD19− CD4− CD8− CD3− NK1.1+) obtained from C57BL/6 CD45.2 mice, and injected into lethally irradiated CD45.2 recipient animals in a ratio of 105 NK cells to 103 HSCs per mouse.
(B) Number of HSCs assessed by FACS. Y axis indicates number of alive Hoechst 33,258− HSCs after overnight incubation. X axis indicates culture conditions.
(C) Flow cytometry analysis of recipient mice peripheral blood 9 weeks after transplantation. Y axis indicates the percentage of donor-derived CD45.1/2+ cells. X axis indicates different culture conditions.
(D) Representative flow cytometry plots of recipient mice peripheral blood 9 weeks after transplantation. Plots show CD45.1 versus CD45.2 expression in HSC and HSC + NK transplanted mice. Black box indicates the percentage of CD45.1/2+ donor-derived cells and the gate used to analyze tri-lineage contribution: red box indicates the percentage of myeloid cells (Gr1/CD11b+), blue box the percentage of B cells (B220+), and gray box the percentage of T cells (CD3+). See also Figure S2.
(E) FACS analysis of peripheral blood (PB), BM, and SP isolated from recipient mice 16 weeks after transplantation. Y axes indicate the percentage of donor CD45.1/2+ cells and X axes indicate distinct culturing conditions. Five animals were included in each group. All data represent mean ± SD from one representative experiment out of three. Two-tailed Student's t test was used to assess statistical significance (p values are indicated).