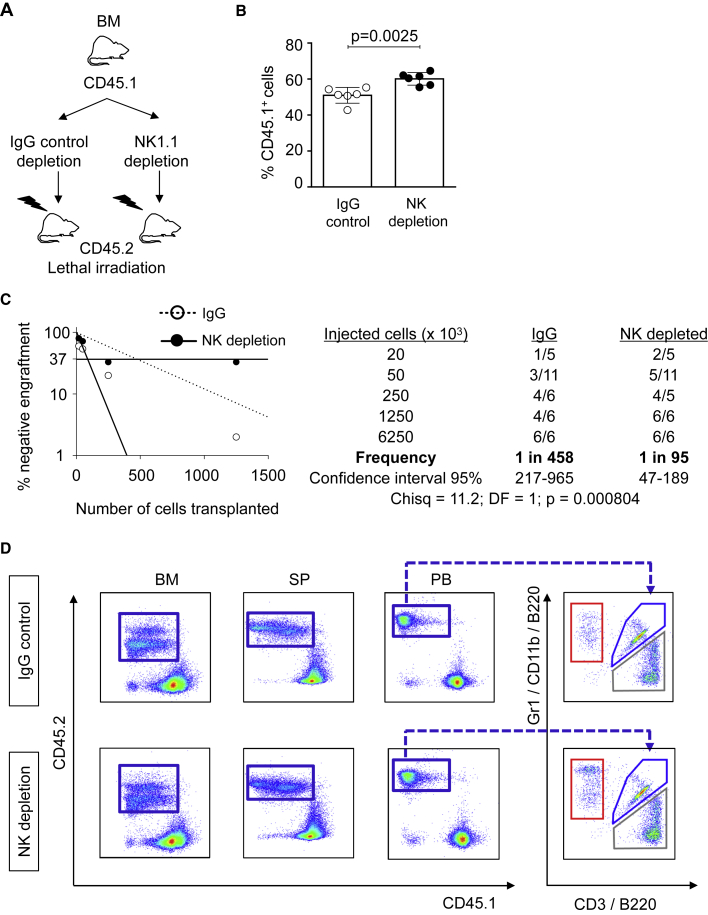
Figure 3.
NK cell depletion from murine BM improves stem cell function
(A) Overview of the experimental strategy for the BM transplantation assay after NK cell depletion (NK1.1) or irrelevant IgG depletion (control). Upon depletion, cells were injected into lethally irradiated CD45.2 animals.
(B) FACS analysis of peripheral blood of recipient mice. Y axis indicates the percentage of donor CD45.1+ cells 9 weeks after transplantation. X axis indicates IgG control or NK cell depletion. In each group 6.25 × 106 cells were transplanted into recipient mice. Six animals were included in each group. All data represent mean ± SD from one representative experiment out of two. Two-tailed Student's t test was used to assess statistical significance (p value is indicated). See also Figure S3.
(C) Panels indicate results from limiting dilution competitive repopulation unit assays. (Left) Logarithmic plot showing the percentage of negative recipients transplanted with different cell doses of murine BM depleted with NK1.1 Ab (black dots) or control IgG Ab (white dots). Only recipients at 16 weeks with engraftment of CD45.1 cells ≥0.1% and contribution to all lineages (T cells, B cells, and granulocytes) higher than 1% were considered responders. (Right) Table showing the number of responders and the total number of recipients transplanted per cell dose. Frequencies of HSCs (1:95 in NK1.1-depleted BM transplants versus 1:458 in IgG-depleted control, p = 0.000804) were calculated according to Poisson statistics using ELDA software based on data from two independent experiments (Chisq, chi-square test).
(D) Representative flow cytometry dot plots showing the percentages of CD45.1+ donor cells (blue boxes, Y axes) and CD45.2+ host cells (X axes). Plots show BM, SP, and PB of mice injected with IgG-depleted control (upper panels) and NK-depleted (lower panels) BM cells 16 weeks after transplant. The panels on the right refer to gated CD45.1+ PB and indicate T (gray box), B (blue box), and myeloid (red box) cells, as determined by the use of antibodies against CD3, B220, and Gr1/CD11b, respectively.