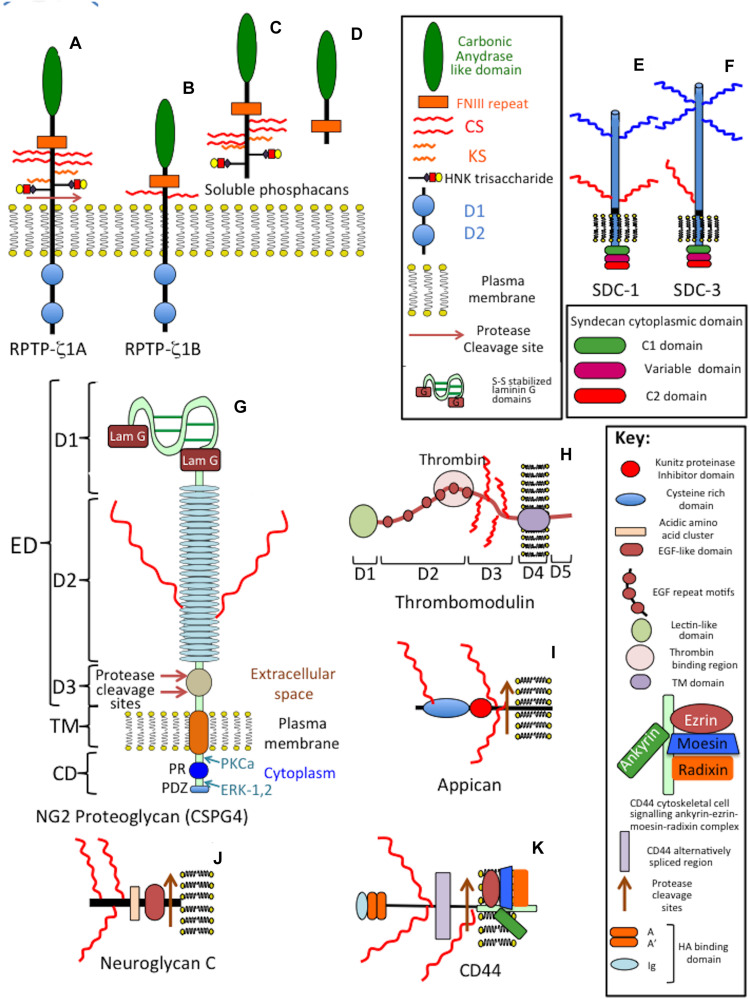
FIGURE 4.
Composite schematic depicting the structural organization of cell membrane attached CS-proteoglycans in the CNS/PNS. Phosphacan precursor, receptor protein tyrosine phosphatase (RPTP)-1A and 1B forms (A,B) and soluble phosphacan released by proteolysis of (A) as shown (C). A non-glycanated truncated phosphacan variant has also been described (D). Syndecan-1 (SDC-1) (E) and syndecan-3 (SDC-3) (F). The transmembrane form of NG2 proteoglycan (CSPG4) (G) and its soluble form released from cells by protease cleavage close to the cell membrane (H). The soluble form of CSPG4 becomes lodged in the ECM through interactions mediated by its LamG N-terminal motifs and by interactions of its central cysteine-rich domain with type IV and VI collagen. Thrombomodulin showing its terminal lectin-like domain, six EGF repeat modules, thrombin binding region which allows it to act as an anti-coagulant in the brain microvasculature and transmembrane cell attachment domain (I). Appican (J), neuroglycan-C (K), and the HA-receptor CD-44 (L) with protease cleavage sites that results in the generation of soluble forms of these proteoglycans. The CS-E chains of appican bind strongly to the growth factors midkine and pleiotrophin, neuroglycan-C binds to ephrin cell surface receptors resulting in induction of cell signaling mediated by the ephrin cytoplasmic regions while sCD44 binds to HA in the ECM through its disulphide stabilized A, A′ and Ig folds. Several CAMs also act as CS-PG receptors through interaction with the CS side chains of these PGs (see Figure 7).