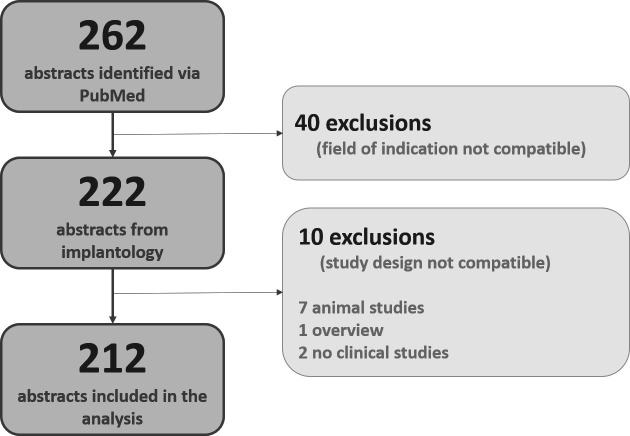
Figure 1.
Description of the selection procedure and documentation of the number of published RCTs from dental implantology, the aim being a data pool to identify—per criterion and study—the degree of adherence to the CONSORT recommendations for abstracts. Fifty study reports had to be excluded from further investigation and analysis because the clinical indication (n=1) or the study design (n=39) were not compatible or the respective data sets referred to investigations of animals (n=7), were reviews (n=1) or were not identifiable as RCTs (n=2). CONSORT, Consolidated Standards of Reporting Trials; RCTs, randomised controlled trials.