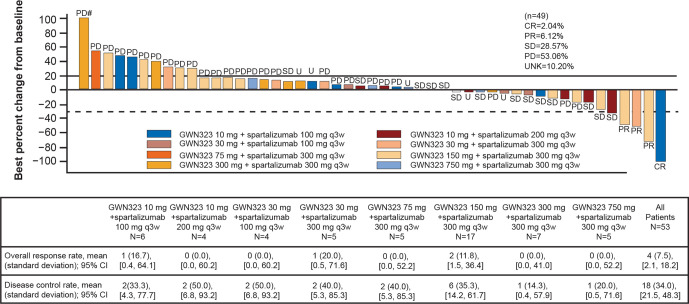
Figure 2.
Best percentage change and best overall response by investigator assessment (RECIST V.1.1) in patients with target lesions in the combination arm. # indicates percentage changes from baseline of >100% are set to 100%. n indicates the number of patients with ≥1 baseline and postbaseline assessment of target lesions. CR, complete response; GWN, GWN323; PD, progressive disease; PR, partial response; q3w, every 3 weeks; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; UNK, unknown.