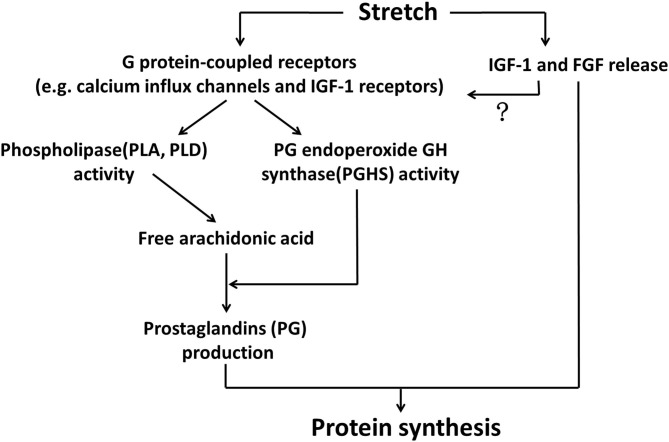
Figure 1.
Stretch-released growth factors and prostaglandins regulated protein synthesis of myotubes. Stretch activated G protein-coupled receptors, such as calcium influx channels and IGF-1 receptors, in the membrane of myotubes, leading to elevated activities of phospholipases (PLA, PLD) and PG endoperoxide GH synthase (PGHS). PLA and PLD liberated arachidonic acid, which was subsequently converted by PGHS into prostaglandins production, such as PGE2 and PGF2. On the other hand, stretching stimuli could also promote growth factors (IGF1 and FGF) that participated in myotube protein synthesis through binding to their specific receptors. Whether these two major mechanisms acted simultaneously to regulate anabolism of myotubes under stretching environment would be a matter of interest.