Figure 3.
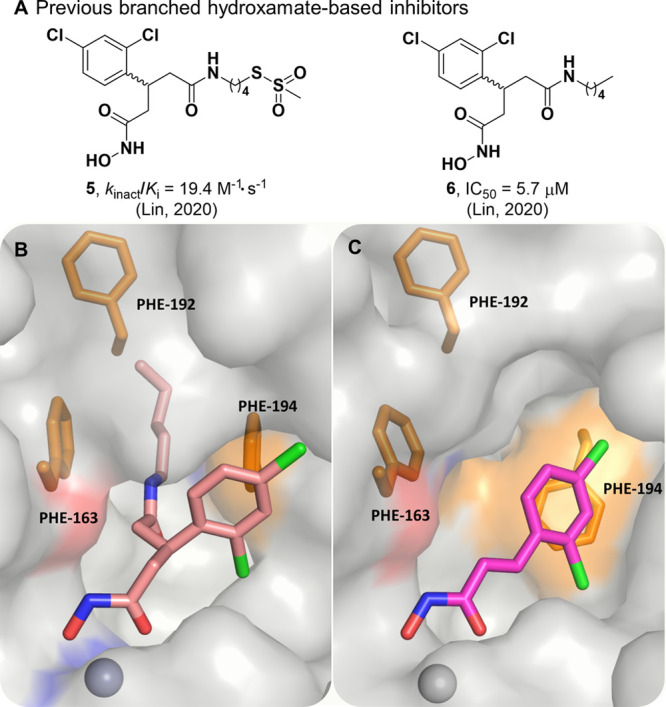
(A) Previous branched hydroxamate-based inhibitors of the BoNT/A LC. (B) X-ray crystal structure of 6 bound in the active site of the LC (PDB 6XCF).27 Accommodation of the alkyl moiety in the hydrophobic subpocket results in disruption of the face-to-face π–π stack between the 2,4-dichlorocinnamic phenyl ring and Phe194. (C) Alternative view of DCHA (purple) bound with the LC. The 2,4-dichlorocinnamic phenyl ring and Phe194 form an offset face-to-face π–π stack.
