Figure 2.
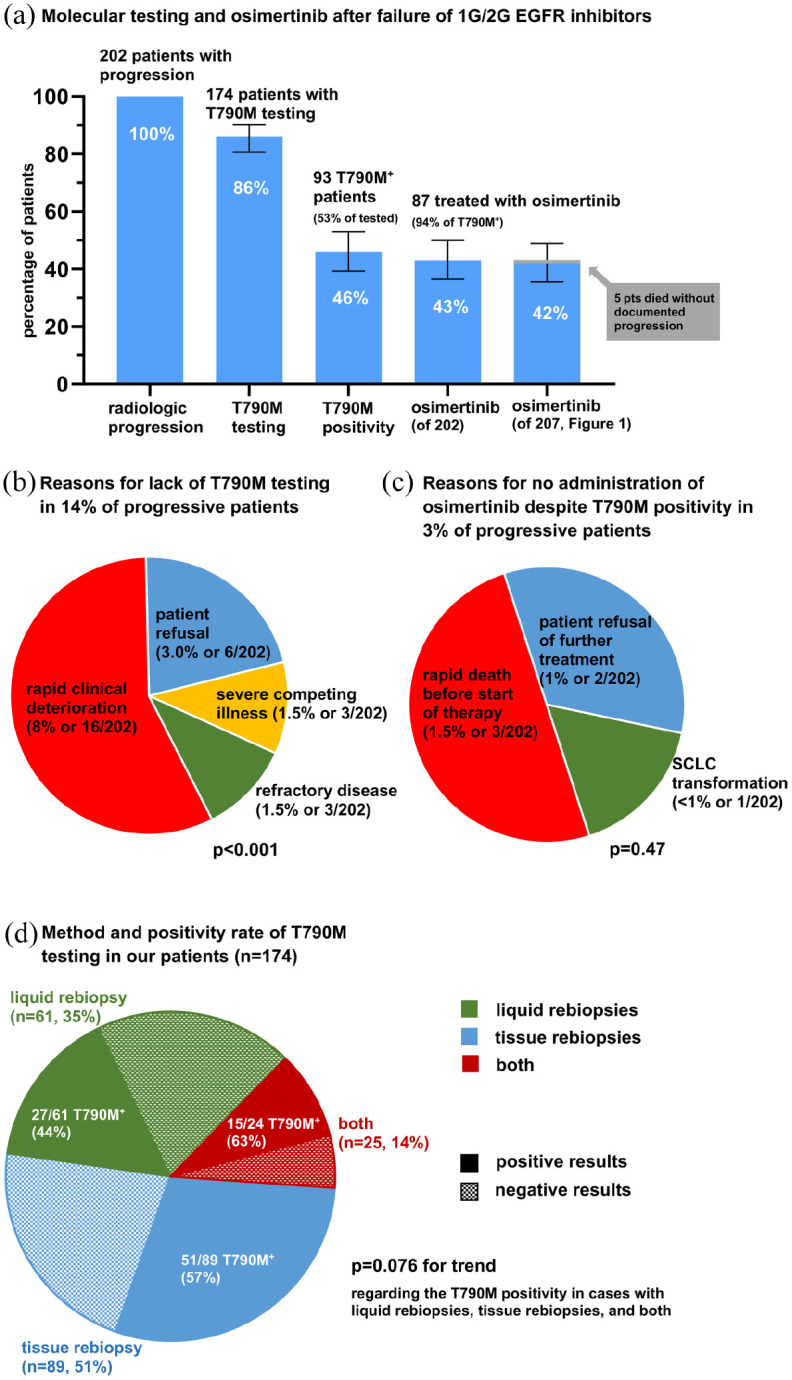
Molecular testing, T790M positivity and next-line osimertinib after failure of 1G/2G-generation EGFR inhibitors in the entire study population. (a) Rate of T790M testing, T790M positivity and next-line osimertinib administration among patients with documented radiologic disease progression under 1G/2G EGFR TKI (n = 202, Figure 1). Error bars indicate 95% CI. (b) Reasons for lack of T790M testing in 14% of progressive patients (chi-square p < 0.001). Patient refusal was due to severe side effects from first-line TKI (5/6), and reluctance to undergo bronchoscopy (1/6). Severe competing illness leading to decision against further anticancer therapy was dementia in two cases, and glioblastoma multiforme in the third. Refractory disease was primary progressive disease at the first restaging after start of 1G/2G EGFR TKI in 2/3 cases, and small-cell transformation at the time of TKI failure in 1/3. (c) Reasons for lack of treatment with osimertinib despite T790M positivity in 3% of progressive patients (chi-square p = 0.47). (d) Utilization and positivity rate of liquid rebiopsies, tissue rebiopsies, and their combination for T790M testing in our patients (chi-square p = 0.076 for trend regarding positivity).
1G/2G, first/second-generation; CI, confidence intervals; EGFR, epidermal growth factor receptor; SCLC, small-cell lung cancer; TKI, tyrosine kinase inhibitors.
