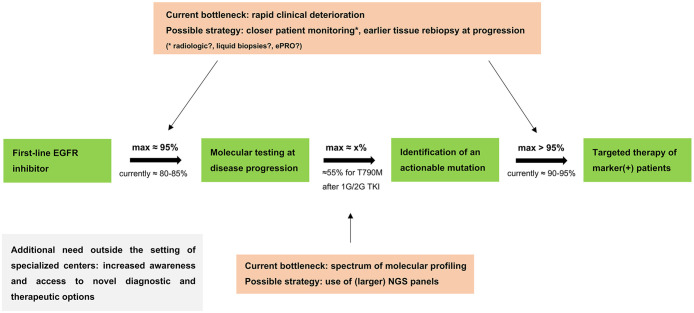
Figure 5.
Implementation of sequential targeted therapies for metastatic EGFR+ NSCLC. Critical parameters, possible improvement strategies and feasibility limit for implementation of sequential targeted therapies in metastatic EGFR+ NSCLC. The maximum testing rate of 95% is taken from Supplemental Table S2 and excludes only patients with EGFR TKI discontinuation due to reasons precluding further treatment (i.e. “other reasons” or patient decision); the actual testing rate of 80–85% is taken from Figure 2a, Supplemental Figure S1a and the literature cited in the Discussion; the actual treatment rate for T790M positive patients with osimertinib of 90–95% is taken from Figure 2a and Supplemental Figure S1A; the maximum treatment rate for T790M positive patients of >95% additionally considers that 3/6 T790M positive patients foregoing osimertinib treatment suffered early death before the drug could be started (Figure 2c), which could potentially have been prevented by an earlier change in therapeutic strategy facilitated by closer patient monitoring; the x% rate of marker-positivity is mutation-specific, for example, approximately 55% for EGFR T790M under 1G/2G EGFR TKI (Figure 2a).21,22 For any next-line targeted therapy, the theoretical upper limit of implementation is the product of these three parameters (0.95*0.95*x), for example, approximately 50% for osimertinib after 1G/2G EGFR TKI. The rate of molecular testing affects the feasibility of implementation for all next-line targeted therapies. This framework appears to be very similar for 1G/2G EGFR inhibitors and osimertinib, as shown in Supplemental Table S2 and explained in the Discussion.
1G/2G TKI, first/second-generation tyrosine kinase inhibitors; EGFR, epidermal growth factor receptor; EGFR+, epidermal growth factor receptor-mutated; ePRO, electronic patient-reported outcomes; NGS, next-generation sequencing; NSCLC, non-small-cell lung cancer.