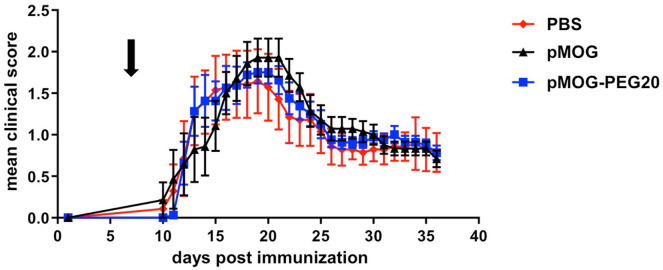
Figure 5.
Therapeutic administration of MOG-peptide 35–55 (pMOG)-polyethylene glycol (PEG)20 does not prevent experimental autoimmune encephalomyelitis (EAE) development, but also does not lead to exacerbation of the disease. Seven days post EAE induction, C57BL/6 mice received phosphate buffered saline (PBS) (control), 7.6 µg pMOG or equimolar amounts of pMOG-PEG20. Mean clinical score per group ± standard error of the mean (SEM) (PBS n = 7; pMOG n = 7; pMOG-PEG20 n = 8). One representative of three independent experiments is shown (further datasets are to be found in Supplemental Figure 8). Adjusted p-values were p = 0.51, 0.59 and 0.99 for PBS versus pMOG, PBS versus pMOG-PEG20, and pMOG versus pMOG-PEG20, respectively, as determined by non-parametric comparison of relative contrast effects.