Figure 1.
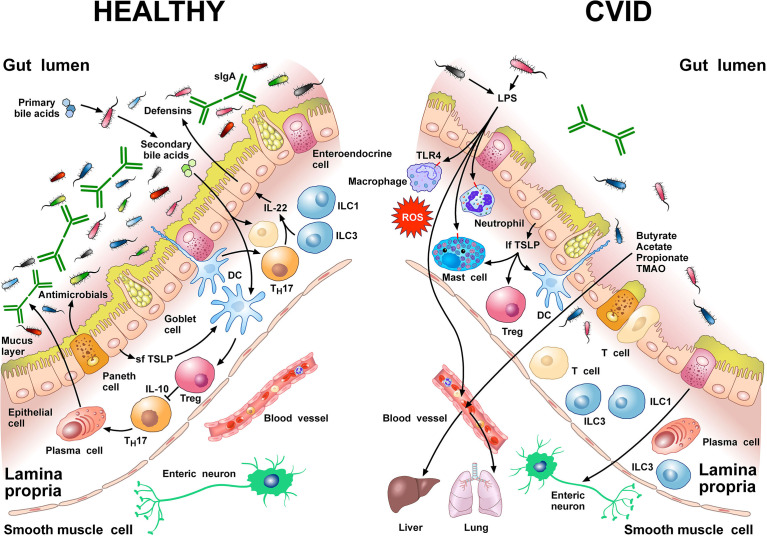
Left side Gut homeostasis and healthy gut are maintained by the interplay between physical barrier (intact mucus layer and epithelial cells) and several cells of the innate and adaptive immune system. In normal subjects four major phyla dominate the gut microbiome: Bacteriodetes, Firmicutes, Proteobacteria, and Actinobacteria. Dendritic cells (DCs) are pivotal for sensing bacterial products and activating antigen-specific CD4+ T cell differentiation. DCs also promote TH17 immunity, Foxp3+ Treg induction, and IgA production by plasma cells (73). Approximately 5% of primary bile acids transit to the colon and can be metabolized by commensal gut flora (74, 75). These secondary bile acids promote Treg generation and modulate the production of cytokines from DCs (76–78). Several other immune cells (e.g., macrophages, mast cells, neutrophils, ILC3, TH1 cells, plasma cells) are sentinels in the mucosal system (79). Neutrophils exert anti-bacterial effects through the release of their stored and newly synthesized mediators and the formation of neutrophils extracellular traps NETs (80). TH17 cells produce IL-22 that promotes secretion of anti-microbial peptides such as β-defensins by epithelial cells. ILC1 and ILC3 are present in human intestinal mucosa (81). ILC3 produces IL-22, which plays a role in containing the commensal flora (82) and protecting epithelial cells (83). Paneth cells produce several molecules with antimicrobial activity (α-defensin, REG3, ANG4, sPLA2) as well as cytokines that can recruit immune cells (84). The short thymic stromal lymphopoietin isoform (sfTSLP), constitutively expressed by human epithelial cells, is crucial in preserving immune tolerance in the gut (85–87). Right side In patients with CVID, alpha and beta diversity of the gut microbiota is reduced compared to healthy donors (22, 66). Repeated or chronic infections damage the intestinal epithelium (44). The disruption of the gut barrier integrity and the reduction of secretory IgA (54) increase microbial translocation (88) and the permeability of pathogen-associated molecular patterns (PAMPs) such as LPS (22, 66, 89). LPS activates TLR4 on human macrophages (90), neutrophils (91), and mast cells (92, 93) to release pro-inflammatory mediators and ROS. The numbers of ILC3 and ILC1 are abnormally high in the inflamed intestinal mucosa (94). The long TSLP isoform (lfTSLP), induced by several components of gut microbiota, exerts pro-inflammatory effects and contributes to intestinal damage (85–87). The passage of bacteria-derived products (e.g., LPS, butyrate, acetate propionate, TMAO) into the circulation is one of the means of communication between the gut microbiota and the lung or the liver (2, 89, 95, 96). The three most common short chain fatty acids (SCFAs) (butyrate, acetate, and propionate) can also exert immunomodulatory/anti-inflammatory roles (97, 98).