Figure 7.
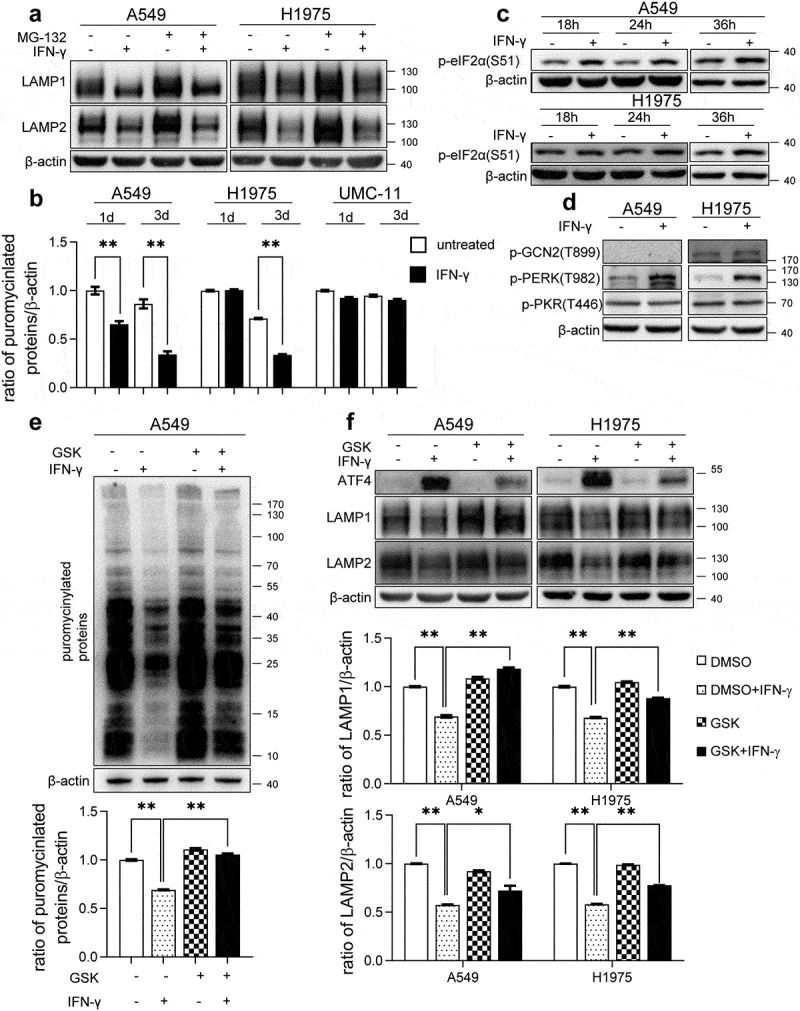
IFN-γ-induced activation of PERK-eIF2α is responsible for the reduction in the expression of LAMP. (a) The indicated cells were treated with IFN-γ or mock treated for 3 days followed by culturing with 10 μM MG132 for an additional 4 h. Immunoblots showing LAMP-1 and LAMP-2 expression. (b) The indicated cells were treated with IFN-γ or mock treated. Puromycin (10 µg/mL) was added during the last 10 min of incubation. Puromycinylated proteins were detected using western blotting and the bar graphs show densitometric analysis of the changes in the abundance of puromycinylated proteins normalized to β-actin for loading variability. **, p < .01. (c) Phosphorylated eIF2α was determined using western blot analyses of A549 and H1975 cells treated with or without IFN-γ (1000 IU/mL) for the indicated time intervals. (d) A549 and H1975 cells were treated with or without IFN-γ (1000 IU/mL) for 12 h. The expression levels of phosphorylated PERK, GCN2, and PKR were determined using western blot analysis. (e) A549 cells were treated with 1000 IU/mL of IFN-γ in the presence of 500 nM GSK2606414 for 24 h. Puromycin (10 µg/mL) was added during the last 10 min of incubation. Puromycinylated proteins were detected using western blotting and the bar graphs show densitometric analysis of the changes in the abundance of puromycinylated proteins normalized to β-actin for loading variability. **, p < .01. (f) The indicated cells were treated with 1000 IU/mL of IFN-γ in the presence of 500 nM GSK2606414 for 2 days. Immunoblots showing ATF-4, LAMP-1, and LAMP-2 expression. LAMP-1 and LAMP-2 expression was normalized to β-actin level for densitometric analyses and is presented as the mean ± SEM. *, p < .05; **, p < .01
