Figure 9.
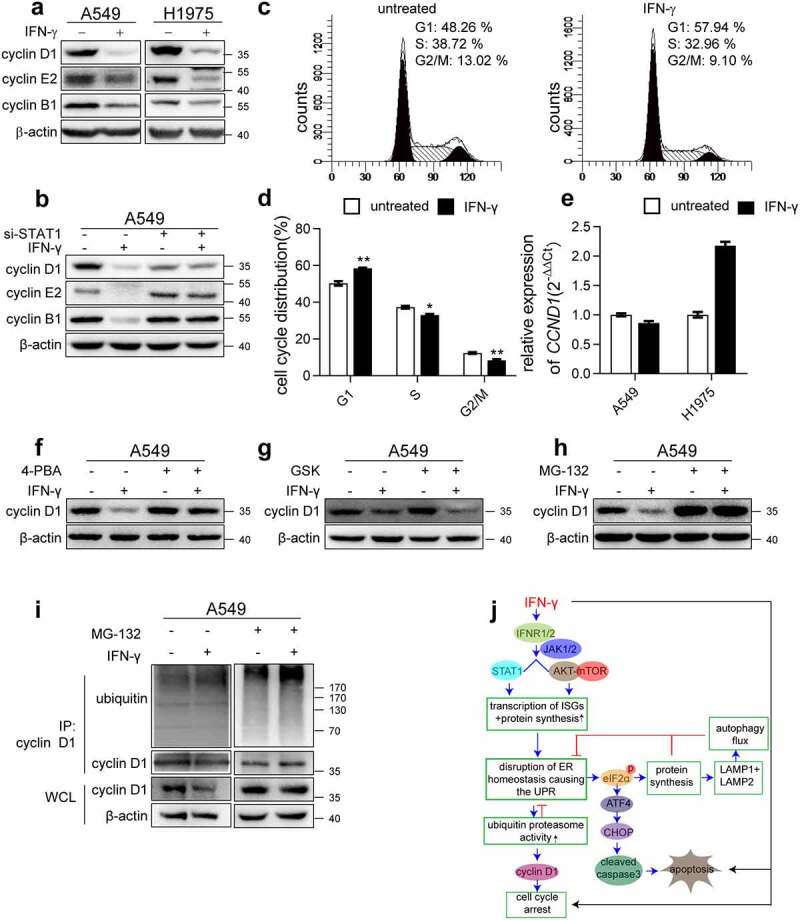
ER stress is involved in IFN-γ-mediated suppression of cell cycle progression. (a) Immunoblots showing cyclin D1, cyclin E2, and cyclin B1 expression in the indicated cells treated with IFN-γ vs. mock treated for 3 days. (b) The indicated cells transfected with control siRNA or si-STAT1 were treated with IFN-γ (1000 IU/mL) for 3 days. Immunoblots showing cyclin D1, cyclin E2, and cyclin B1 expression. (c) A549 cells were treated with IFN-γ (1000 IU/mL) for 24 h, and PI staining was performed to determine cell cycle progression, and (d) the data are presented as the means ± SEM from 3–4 individual experiments. (e) The expression of CCND1 was assessed using qRT-PCR in the indicated cells after treatment with IFN-γ (1000 IU/mL) or mock-treated for 24 h. (f) Cyclin D1 expression was assessed in A549 cells treated with IFN-γ in the presence or absence of 4-PBA. (g) A549 cells were treated with IFN-γ (1000 IU/mL) for 3 days in the presence of GSK2606414. Cyclin D1 expression was determined using western blotting. (h) A549 cells were treated with IFN-γ (1000 IU/mL) for 3 days followed by culturing with MG132 (10 μM) for an additional 4 h. Cyclin D1 expression was determined using western blotting. (i) Cell lysates were immunoprecipitated with anti-cyclin D1 antibody, and ubiquitination was detected using western blotting. (j) Schematic depicting the effect of IFN-γ signaling on lung adenocarcinoma cells. IFN-γ induces ER stress and UPR in lung adenocarcinoma cells through the activation of JAK1/2-STAT1 and AKT-mTOR signaling. This UPR consequently reduced LAMP-1 and LAMP-2 expression and led to impairment of autophagic flux. We also demonstrated that IFN-γ-induced ER stress contributes to IFN-γ-triggered apoptotic cell death and cell cycle arrest
