Figure 2.
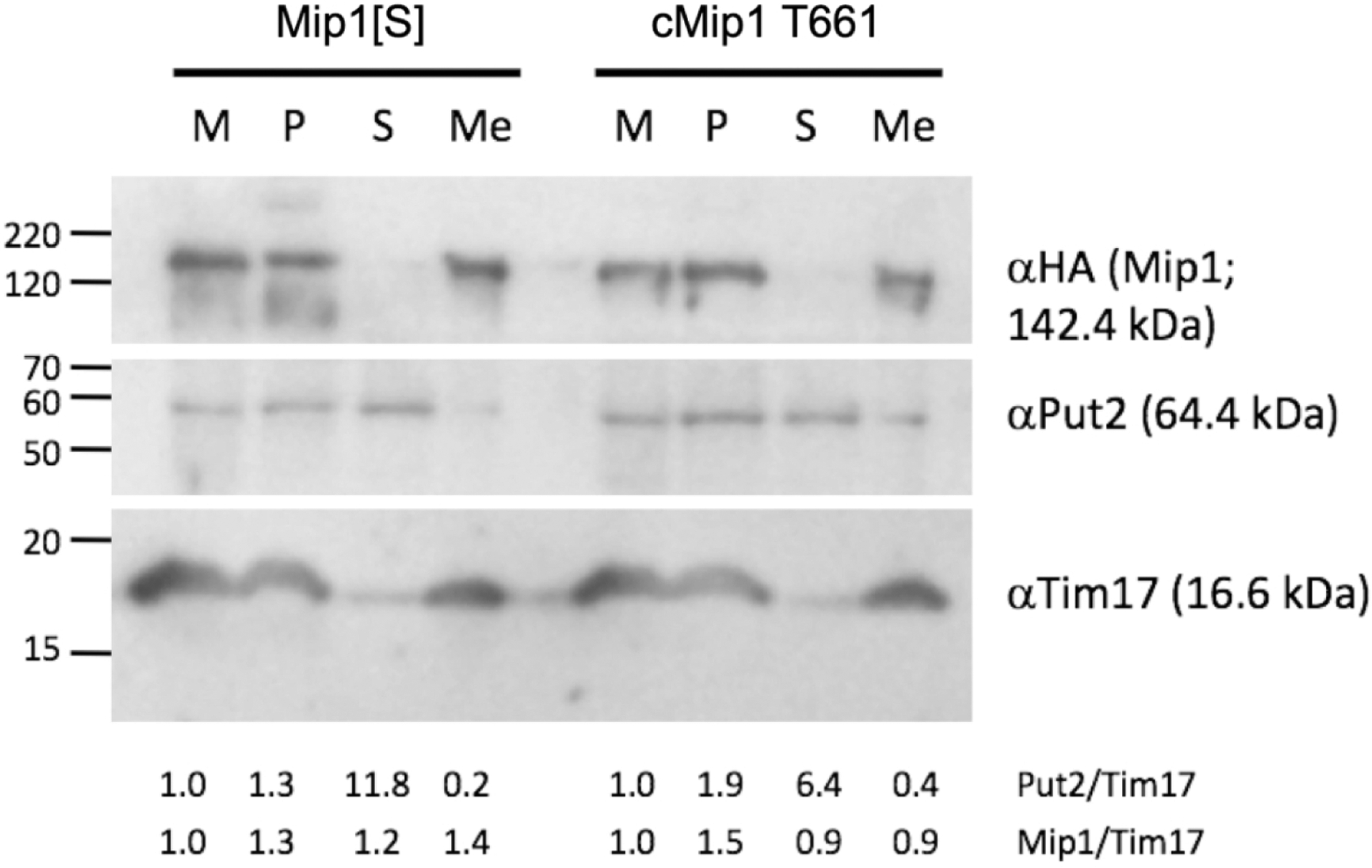
Submitochondrial fractionation and localization of Mip1. Mitochondria resuspended in hypotonic lysis buffer before homogenization and centrifugation (M); corresponding amounts of mitochondrial pellet obtained after homogenization and centrifugation (P); supernatant after homogenization and centrifugation (S); and mitochondrial membrane fractions (Me) isolated via sucrose gradient centrifugation were run on a 12% SDS-PAGE gel, transferred to nitrocellulose and subjected to immunodetection with: 1) anti-HA mouse monoclonal antibody to detect the HA-tagged Mip1[S] and cMip1 T661 variants (top of blot); antibodies against the mitochondrial matrix protein Put2 (middle panel) and the mitochondrial inner membrane protein Tim17 (lower panel). Molecular weight markers on the left-hand side are indicated in kDa. The ratio of Put2 to Tim17 (Put/Tim) and Mip1 to Tim17 (Mip/Tim) are presented below the blot. The amount of Put1 and Mip1 for each of Mip1[S] and cMip1 T661 in the M fraction was set to 1.
