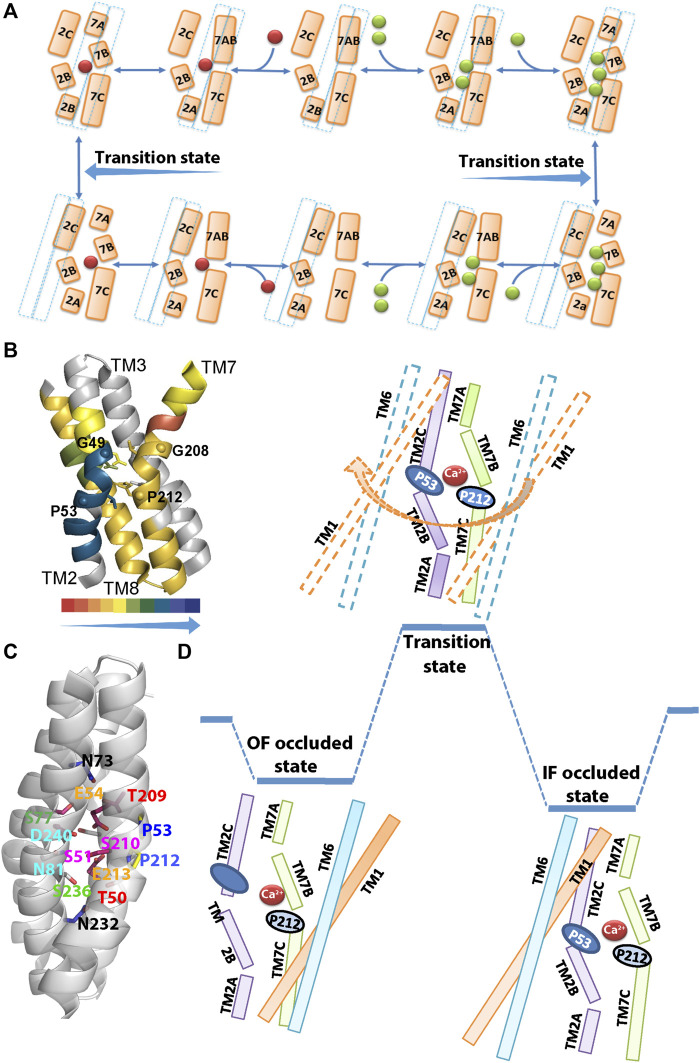
FIGURE 2.
Structural organization of ion binding sites in NCX_Mj. (A) The Na+/Ca2+ exchange cycle involves a separate translocation of the 1Ca2+- and 3Na+-bound species. Green and red spheres denote the Na+ and Ca2+ ions, respectively, whereas a dashed line denotes the sliding cluster (TM1/TM6). According to this model, the transition state is situated between the two occluded states, where the sliding event of the TM1/TM6 cluster takes place during the transition state and it is associated with the OF/IF swapping in either direction. (B) The backbone dynamics of the four ion-coordinating helices of NCX_Mj are represented by the overlay of the HDX-MS hit maps (Giladi et al., 2017) on the crystal structure of 3Na+-bound NCX_Mj (5HXE). The arrow direction indicates the increased rigidity of the backbone dynamics (the color key shows the percentage of deuterium incorporation). Notably, the backbone dynamics of TM2B is much more constrained than the other ion-coordinating segments (TM2C, TM7B, TM7C, and TM8A). Thus, the helix-breaking residues (G49, P53, G208, and P212) belonging to the GTSLPE repeats (allocated at the interface of the TM2B/TM2C and TM7B/TM7C segments) may specifically shape the local backbone dynamics at “catalytic” ion-coordinating residues. (C) Single-point mutations of residues allocated at TM2B, TM2C, TM3A, TM7B, TM7C, and TM8A differentially affect the rate-equilibrium relationships of bidirectional ion movements (Giladi et al., 2016a; Giladi et al., 2016b; Giladi et al., 2016c; van Dijk et al., 2018). Inversely matched pair residues (shown in the same colors) differentially affect the ion transport activities, thereby suggesting that the functional asymmetry of inversely located pair residues occurs at the single residue level. (D) The occluded and transition states are schematically represented in the case of Ca2+. In the Ca2+ occluded states the hydrophilic gap between the TM2C (P53) and TM7B (P212) segments becomes closer to each other for a hydrophobic patch, although the HDX-MS data reveal that TM2C, TM7B, TM7C, and TM8A, nearby the hydrophobic patch, remain quite flexible upon ion occlusion (Giladi et al., 2017). After the ion occlusion, the hydrophobic patch may undergo further conformational adjustments through the interactions of “catalytic” residues (E54, E213, D240, S51, S77, and T209) with Ca2+. As a result, the sliding of the TM1/TM6 cluster can take place to accomplish the OF/IF swapping (the movement of the TM1/TM6 cluster during the transition state is denoted by an arrow). The present considerations may represent the basis for future MD simulations.