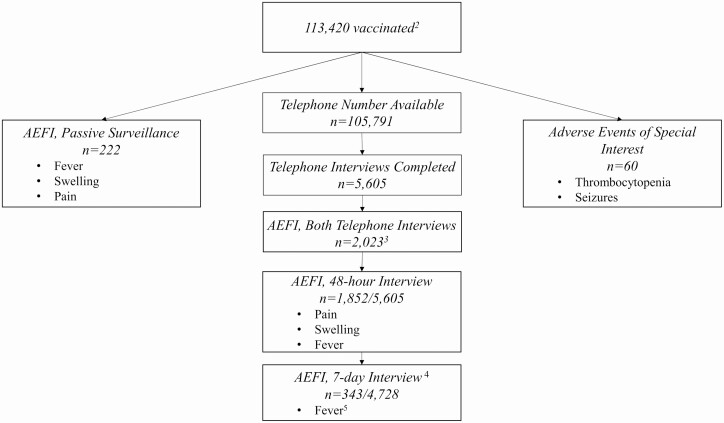
Figure 1.
Most common safety-related events by assessment method among individuals who were reported to have received TCV1—Navi Mumbai, India, 2018. 1Typbar-TCV, Bharat Biotech International Limited, India. 2Per administrative reports. 3A total of 2023 children experienced at least 1 AEFI at either time point (48 hours or 7 days following TCV vaccination). At 7 days following vaccination, 172 of 343 children had reported an AEFI at 48 hours as well. 4Telephone interviews were conducted among 5605 caregivers of TCV recipients at 48 hours after vaccination. At 7 days following TCV vaccination, 4728 of those caregivers were interviewed again. 5Fever was the only AEFI reported among more than 1% of vaccine recipients 7 days following vaccination. Abbreviations: AEFI, adverse events following immunization; TCV, typhoid conjugate vaccine.