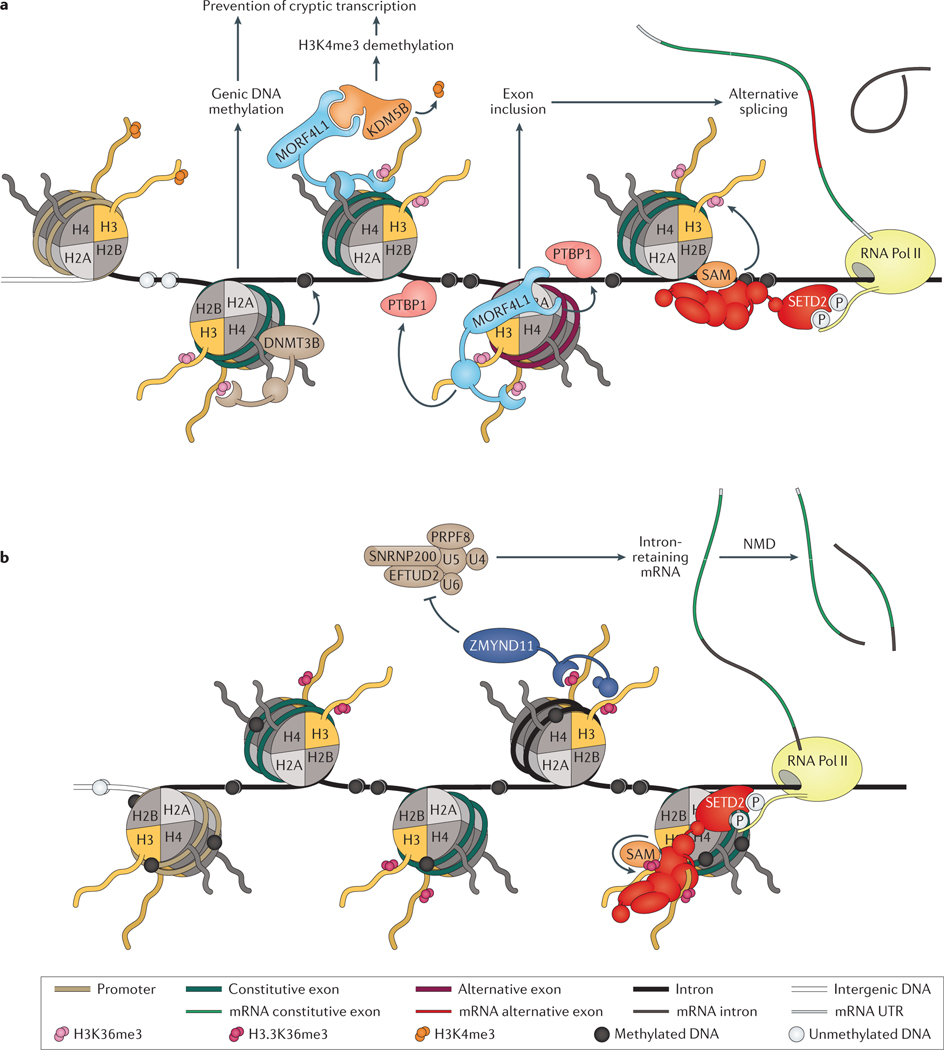
Figure 4 |. Maintenance of transcriptional fidelity.
a | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) mediates gene body DNA methylation through its interaction with trimethylated lysine 36 on histone H3 (H3K36me3) via its PWWP domain. DNMT3B can prevent spurious transcriptional initiation by promoting the DNA methylation of intragenic transcription factor binding motifs, which can influence the binding of some transcription factors. Mortality factor 4-like protein 1 (MORF4L1; also known as MRG15) also binds to H3K36me3 and can recruit lysine-specific demethylase 5B (KDM5B), the trimethylated lysine 4 on histone H3 (H3K4me3) lysine demethylase. The activity of KDM5B prevents spurious transcription initiation by removing H3K4me3, which is associated with active gene promoters. In addition, MORF4L1 can mediate gene splicing, whereby MORF4L1 recruits polypyrimidine tract-binding protein 1 (PTBP1) to DNA to promote exon inclusion in the mature mRNA transcript. b | A histone H3K36me3-specific reader, zinc finger MYND domain-containing protein 11 (ZMYND11; also known as BS69), mediates intron retention of certain target genes. ZMYND11 contains a bromo-PWWP domain that specifically binds trimethylated lysine 36 on the histone H3.3 variant (encoded by the H3F3A or H3F3B genes), rather than the canonical histone H3.1 or H3.2. Incorporation of the H3.3 variant histone into chromatin is independent of DNA synthesis, and it is deposited at distinct genomic regions, including gene bodies, telomeres, and pericentric chromatin. Histone H3.3 phosphorylation at serine 31 abrogates ZMYND11 binding to H3.3K36me3, suggesting that ZMYND11 recognition of H3.3K36me3 is regulated by signalling pathways that mediate histone H3.3 serine 31 phosphorylation. ZMYND11 inhibits the activity of the U5 small nuclear ribonucleoprotein particle protein complex — which comprises U5 small nuclear ribonucleoprotein 40 kDa protein (U5), 116 kDa U5 small nuclear ribonucleoprotein component (EFTUD2), pre-mRNA-processing-splicing factor 8 (PRPF8), U5 small nuclear ribonucleoprotein 200 kDa helicase (SNRNP200), U4 (RNU4–1), and U6 (RNU6–1) — to promote intron retention, which can trigger the nonsense mediated decay (NMD) pathway. The NMD surveillance pathway usually degrades erroneous transcripts, which contain premature a stop codon, and in this way, can reduce gene expression and could regulate transcriptional output.