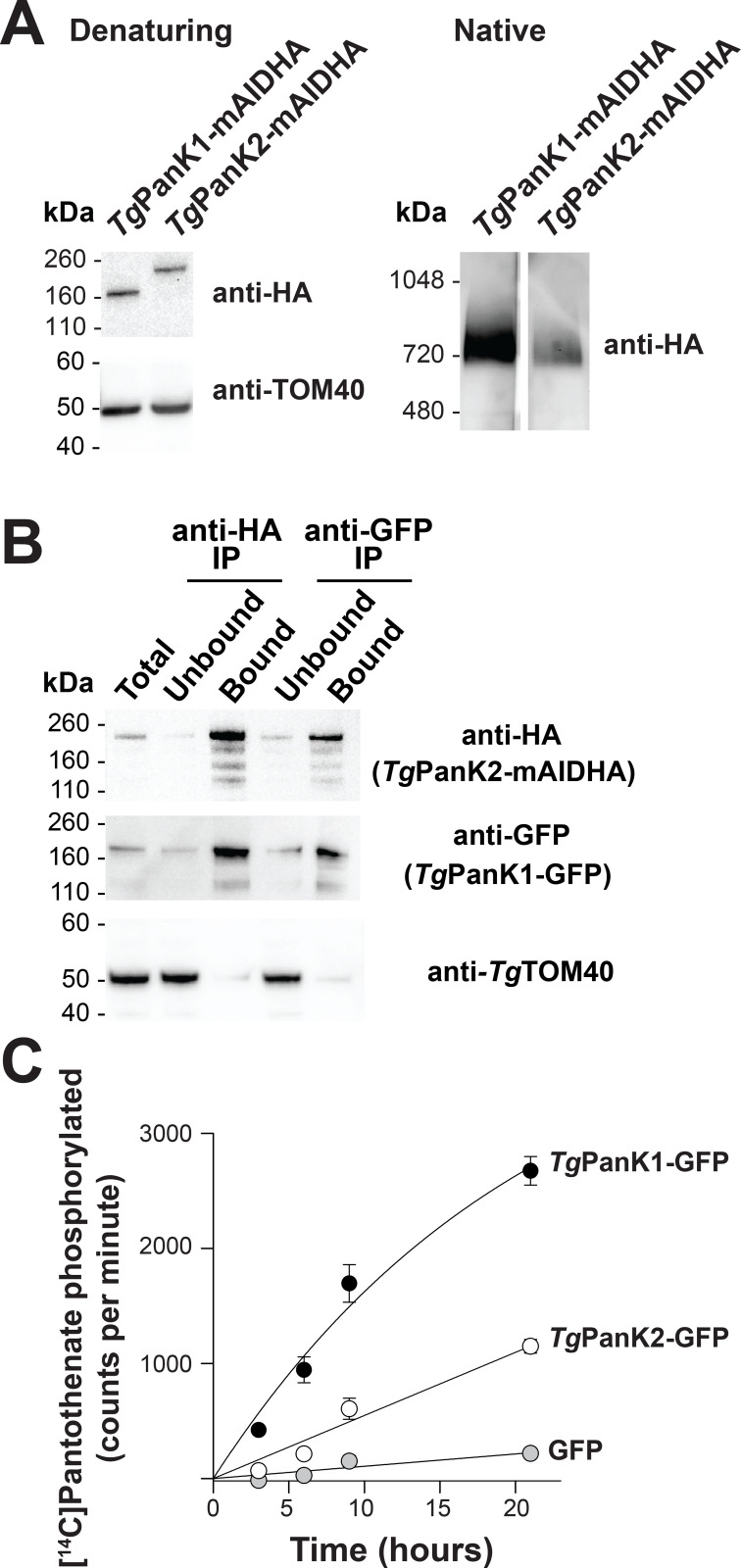
Fig 3. TgPanK1 and TgPanK2 are part of a single protein complex with PanK activity.
(A) Denaturing and native western blot analyses of the HA-tagged proteins in TgPanK1-mAIDHA and TgPanK2-mAIDHA parasite lines. The expected sizes of TgPanK1-mAIDHA and TgPanK2-mAIDHA are ~143 kDa and ~189 kDa, respectively. Western blots were performed with an anti-HA antibody and each blot shown is a representative of three independent experiments, each performed with different batches of parasites. Denaturing western blots were also probed with anti-TgTOM40, which served as a loading control. (B) Western blot analysis of proteins from TgPanK1-GFP/TgPanK2-mAIDHA parasite lysates immunoprecipitated with GFP-Trap and anti-HA beads (TgPanK1-GFP is 160 kDa). Protein samples were collected before immunoprecipitation (Total), from the fraction not bound to the GFP-Trap/anti-HA beads (Unbound), and from the fraction bound to the GFP-Trap/anti-HA beads (Bound). Membranes were probed with anti-GFP and anti-HA antibodies, and the blot shown is representative of three independent experiments, each performed with different batches of parasites. TgTOM40 served as a control protein that is part of an unrelated protein complex. Bound fractions contain protein from 4 × as many cells as the total and unbound lanes. (C) The phosphorylation of [14C]pantothenate (initial concentration 2 μM) over time by protein samples immunoprecipitated with GFP-Trap from TgPanK1-GFP/TgPanK2-mAIDHA (black circles),TgPanK1-HA/TgPanK2-GFP (white circles) and untagged GFP (grey circles) lines. Data shown are representative of two independent experiments, each performed with a different batch of parasites and carried out in duplicate. Error bars represent the range/2 and are not shown if smaller than the symbols.