Abstract
An efficient composite was constructed based on aminated chitosan (NH2Cs), attapulgite (ATP) clay and magnetic Fe3O4 for adsorptive removal of Cr(VI) ions. The as-fabricated ATP@Fe3O4-NH2Cs composite was characterized by Fourier Transform Infrared Spectroscopy (FTIR), Thermal Gravimetric Analyzer (TGA), Scanning Electron Microscope (SEM), Zeta potential (ZP), Vibrating Sample Magnetometer (VSM), Brunauer–Emmett–Teller method (BET) and X-ray photoelectron spectroscope (XPS). A significant improve in the adsorption profile was established at pH 2 in the order of ATP@Fe3O4-NH2Cs(1:3) > ATP@Fe3O4-NH2Cs(1:1) > ATP@Fe3O4-NH2Cs(3:1) > Fe3O4-NH2Cs > ATP. The maximum removal (%) of Cr(VI) exceeded 94% within a short equilibrium time of 60 min. The adsorption process obeyed the pseudo 2nd order and followed the Langmuir isotherm model with a maximum monolayer adsorption capacity of 294.12 mg/g. In addition, thermodynamics studies elucidated that the adsorption process was spontaneous, randomness and endothermic process. Interestingly, the developed adsorbent retained respectable adsorption properties with acceptable removal efficiency exceeded 58% after ten sequential cycles of reuse. Besides, the results hypothesize that the adsorption process occurs via electrostatic interactions, reduction of Cr(VI) to Cr(III) and ion-exchanging. These findings substantiate that the ATP@Fe3O4-NH2Cs composite could be effectively applied as a reusable adsorbent for removing of Cr(VI) ions from aqueous solutions.
Subject terms: Chemistry, Materials science
Introduction
Indeed, the recent prosperity of industries has obvious positive impacts on economic growth; however, disposal of the industrial effluents into the water bodies without reasonable treatment can undoubtedly eradicate mankind1,2. One of the most toxic pollutants that pose jeopardy to human health as well as the entire environment is hexavalent chromium (Cr(VI)). Therefore, Cr(VI) has been deemed to be carcinogenic, mutagenic and displayed higher toxicity due to its enormously mobile in the surroundings3,4. Subsequently, the World Health Organization has authorized that the maximum limit of Cr(VI) in potable water should not exceed 0.05 mgL−15,6. Despite these mentioned risks, there are number of significant industries mainly based on Cr(VI) including steel, textile, dyeing, cement, electroplating and leather tanneries7,8. Therefore, several researches have been focused on the removal of Cr(VI) ions from their aqueous solutions via diverse techniques including coagulation9, chemical precipitation10, adsorption11,12, membrane separation13, catalysis14, ion exchange15 and electrodialysis16. Principally, there are strict criteria to select the appropriate removal technique such as low energy consumption, process simplicity, renewability and the low operational cost17. Accordingly, adsorption can be considered as the most preferred technique to remove Cr (VI) from aqueous solution18,19. Consequently, a plethora of the adsorbents such as activated carbon, clay materials, polymers, natural products, and metal/mixed oxide nanoparticles have been used for the removal of Cr(VI) from wastewater20,21.
Chitosan (Cs) is a cationic polysaccharide polymer that is easily obtained via N-deacetylation of chitin, the essential component of the exoskeleton of crustaceans like shrimp, fungi, crab and insects22,23. Owing to its unparalleled merits such as biocompatibility, polyelectrolyte properties, recyclability, hydrophilicity, biodegradability and adhesion properties, chitosan has a significant deal of interest as an efficient cationic adsorbent for removal of heavy metals, pharmaceutical pollutants and organic dyes from their aqueous solutions24,25. Besides, the presence of the chemically active groups in the chitosan structure such as amino and hydroxyl groups enable the formation of chitosan-derivatives with worthy properties by Schiff base formation, grafting, carboxymethylation, and amination26–28. For instance, aminated chitosan is a newly-established chitosan derivative with extra amine groups, which is expected to enrich the adsorption characteristics of the native chitosan29,30. Although all these features of chitosan, it possesses serious drawbacks including low adsorption kinetic, low surface area, high tendency to agglomerate, poor mechanical strength and low adsorption capacity31,32.
Incorporation of clays into chitosan matrices is a feasible solution to overcome its flaws since clays have great features; good adsorptive properties, low cost, high thermal stability, high surface area and special catalytic activity33–35. Amongst these clays, attapulgite is a subset of hydrous magnesium aluminum silicate with concrete features including hydrophilicity, non-toxicity, low price, abundant resources as well as its high surface area and high porosity36,37. Therefore, attapulgite has been vastly utilized in vital fields such as agriculture, catalysis, anticorrosion and wastewater treatment38–40.
Based on aforementioned interests, an attempt was made in this study to fabricate a new adsorbent composite for efficient adsorptive removal of Cr(VI) ions from their aqueous solutions with a highly adsorption performance and better recyclability. Taking advantages of NH2Cs derivative and ATP clay as well as to allow their beneficial adsorption features to be combined. Herein, ATP@Fe3O4-NH2Cs magnetic composite was successfully synthetized and well-characterized using several analyses tools. Moreover, the aptitude adsorption of the developed adsorbent toward of Cr(VI) ions was achieved using a batch adsorption technique under several studied conditions. Furthermore, isotherms, kinetics and thermodynamics studies were thoroughly studied. Besides, the ability of the developed adsorbent composite to be reuse for ten consecutive adsorption cycles was also examined.
Experimental section
Materials
Chitin (degree of acetylation = 0.94) was purchased from Daejung (Korea), Potassium dichromate (Assay ≥ 99%), Para-benzoquinone (PBQ; 99%), Iron chloride hexahydrate (≥ 99%) and Ferrous chloride tetrahydrate (≥ 99%) were delivered from Sigma-Aldrich (Germany). Ethylenediamine (EDA; 99%), Glutaraldehyde (25%) and Sodium hydroxide (98%) were brought from Aladdin Industrial Corporation (China). Attapulgite (ATP) was supplied from the Huaiyuan Mining Industry Co., Ltd. (China). Hydrochloric acid (37%), Ammonium hydroxide (99%) and Acetic acid (98%) were acquired from Loba Chemie (India).
Synthesis of magnetite Fe3O4
Magnetic Fe3O4 was made-up by co-precipitation technique41. Exactly, FeCl3·6H2O (0.092 mol) and FeCl2·4H2O (0.046 mol) were dissolved into 300 mL distilled H2O water under N2 atmosphere. After that, ammonia solution (25%) was slowly added to the reaction solution until pH reaches 9 and then the solution kept under magnetic stirring for 80 min at 70 °C. Ultimately, the formed particles were separated utilizing an external magnet, washed with ethanol and ultimately dried at 60 °C for 12 h.
Synthesis of aminated chitosan (NH2Cs ) derivative
NH2Cs was synthesized according to the authors preceding work with a slight modification42.Transformation of chitin to NH2Cs was achieved via three main steps. The first step involves the activation of –OH− groups of chitin in which 8 g of chitin was soaked into PBQ solution (6.9 mM; pH 10) which acts as an activator agent. The reaction mixture was conducted under continuous stirring for 6 h at 60 °C. The resultant activated chitin was washed with distilled H2O to remove the excess of PBQ molecules. The second step includes the formation of amino-chitin, since, and followed by dispersion in EDA solution (6.9 mM) for 6 h under constant stirring at 60 °C. The obtained aminated chitin was separated and washed several times using distilled H2O to remove the unreacted EDA molecules. Finally; the third step involves deacetylation of aminated chitin which was achieved by immersing it in NaOH (50%) solution for 22 h under magnetic stirring at 140 °C. The gotten aminated chitosan (NH2Cs) was filtrated, washed with distilled H2O and dried at 60 °C.
Fabrication of ATP@Fe3O4-NH2Cs composite
A specific amount of NH2Cs was dissolved into 20 mL of acetic acid (2%; v/v) aqueous solution under ultrasonic stirring for 45 min. Next, 0.02 g of Fe3O4 was slowly added into NH2Cs solution, and then kept under vigorous stirring for 90 min until the reaction solution became totally homogenous. Then after, an appropriate amount of ATP clay was added to the reaction mixture, and followed by adding 4 mL of glutaraldehyde (25%; v/v) solution. The composite mixture was left under continuous stirring at 60 °C for another 90 min. lastly, the resultant composite was separated, washed with ethanol and left overnight for drying at 45 °C. The ATP@Fe3O4-NH2Cs composite was prepared with different weight ratios of ATP and Fe3O4-NH2Cs composite namely; ATP@Fe3O4-NH2Cs (1:3), ATP@Fe3O4-NH2Cs (1:1) and ATP@Fe3O4-NH2Cs (3:1), respectively.
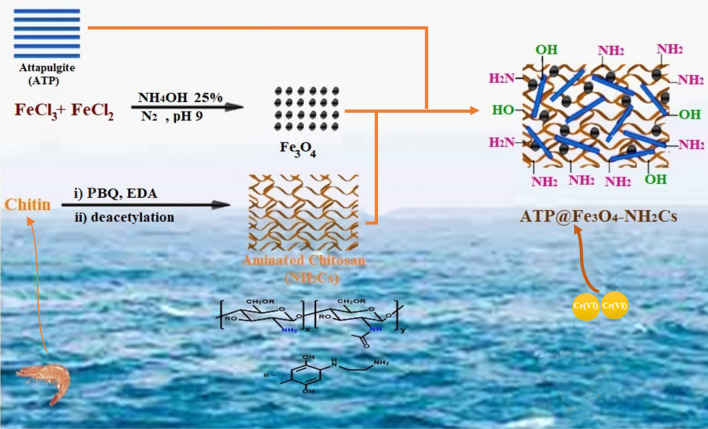
A schematic representation for the fabrication of ATP@Fe3O4-NH2Cs magnetic composite was depicted in Fig. 1.
Figure 1.
A schematic representation for the fabrication process of ATP@Fe3O4-NH2Cs magnetic composite.
Characterization
To investigate the surface morphologies of developed ATP@Fe3O4-NH2Cs composite as well as NH2Cs and ATP clay a Scanning Electron Microscope (SEM; Joel Jsm 6360LA, Japan) was employed under a voltage potential of 20 kV. The examined samples were placed on aluminum stumps and coated with a thin layer of gold via a sputter coating system. The thermal stability was examined under nitrogen atmosphere by Thermal Gravimetric Analyzer (TGA; Shimadzu-50, Japan), while the temperature was raised from 10 to 700 °C at constant heating rate of 20 °C/min and flow rate of 40 mL/min. In addition, the chemical composition of ATP@Fe3O4-NH2Cs composite was explored by Fourier Transform Infrared Spectroscopy (FTIR; Shimadzu-8400 S, Japan), while the absorbance was scanned in the wavenumber range 500–4000 cm–1. Besides, a vibrating Sample Magnetometer (VSM-8600, Lake Shore Cryotronics, Inc., USA) was utilized for evaluating the magnetic property, while Zeta potential (Malvern, UK) was utilized to determine the surface charge. X-ray photoelectron spectroscope (XPS, Axis Ultra DLD, Shimadzu, Japan) was employed for examining the elemental-surface composition of the developed adsorbent. Furthermore, the specific surface area was measure using Brunauer–Emmett–Teller method (BET; Beckman Coulter, SA3100, USA).
Adsorption studies
Batch experiments were executed for evaluating the adsorption profile of ATP@Fe3O4-NH2Cs composite. An accurate 0.01 g of ATP@Fe3O4-NH2Cs composite was soaked into 20 mL of Cr (VI) solution with different concentration ranging from 50 to 200 mg/L at a constant stirring speed (200 rpm min−1). To optimize pH medium, pH of Cr (VI) solution was adjusted ranging from 1 to 8 by utilizing an aqueous solution of a strong acid and/or base. Moreover, the effect of ATP@Fe3O4-NH2Cs composite dosage onto adsorption of Cr (VI) was studied in the range 0.001–0.025 g, as well as the temperature effect, was studied in range 25–55 °C. After each experiment, the magnetic adsorbent was separated by an external magnet and the remaining concentration of Cr (VI) was detected via a spectrophotometer at λmax = 540 nm. The adsorption capacity (q) and the removal percent (R%) were calculated from Eqs. 1 and 2, respectively.
| 1 |
| 2 |
where, Co and Ct, are the Cr(VI) initial concentration and its concentration at time t, respectively. While, V and W are the volume of Cr(VI) and the weight of ATP@Fe3O4-NH2Cs composite, respectively.
Reusability test
From the economical point of view, the selection of an efficient adsorbent strongly depends on its recycling characteristic quality. Therefore, recyclability test was executed to assess the reuse aptitude for the ATP@Fe3O4-NH2Cs magnetic composite. In brief, the magnetic ATP@Fe3O4-NH2Cs composite was collected after completion the adsorption process by an exterior magnet, and followed by immersing in 25 mL of the desorption medium comprising of Methanol/NaCl solution mixture under stirring for 1 h. After complete the desorption process, ATP@Fe3O4-NH2Cs composite was separated magnetically for reuse for ten consecutive cycles.
All experiments were conducted in triplicate, and the results obtained were represented as the means corrected by standard deviation (± S.D.).
Results and discussion
Adsorbent characterization
FTIR
Figure 2 shows FTIR spectra of Fe3O4, NH2Cs, ATP and ATP@Fe3O4-NH2Cs composite. The spectrum of Fe3O4 reveals absorption broad at 3437 cm−1 which is ascribed to stretching vibration of –OH− group43. In addition, the detected bands at 1639 and 892 cm−1 are assigned to –OH− bending and vibrating modes, correspondingly44. Furthermore, the observed bands at 557 and 1405 cm-1 are related to Fe–O stretching45. FTIR spectrum of NH2Cs points out absorption bands at 2901, 2216 and 1619 cm−1 which correspond to CH2, COH stretching and N–H bending vibrations, respectively46. Besides, the broad bands at 3441 and 1062 cm−1 which are ascribed to stretching vibration of –OH− and C–N groups, respectively. Additionally, there are two bands at 2907 and 1402 cm−1 which attributed to C–H stretching vibration and in-plane bending vibration, respectively31. The spectrum of ATP shows a peak at 3563 cm-1 belongs to M–OH bonds stretching vibration, where M; Si, Mg and Al. Also, bands at 3404 and 1653 cm−1 are related to vibrations of OH bending and OH stretching, respectively36. Moreover, the band at 484 cm−1 is ascribed to Si–O–Si bond binding vibration39. FTIR spectrum of ATP@Fe3O4-NH2Cs composite elucidates the fundamental peaks of the pristine materials (i.e. Fe3O4, NH2Cs and ATP), inferring the successful fabrication of ATP@Fe3O4-NH2Cs composite.
Figure 2.
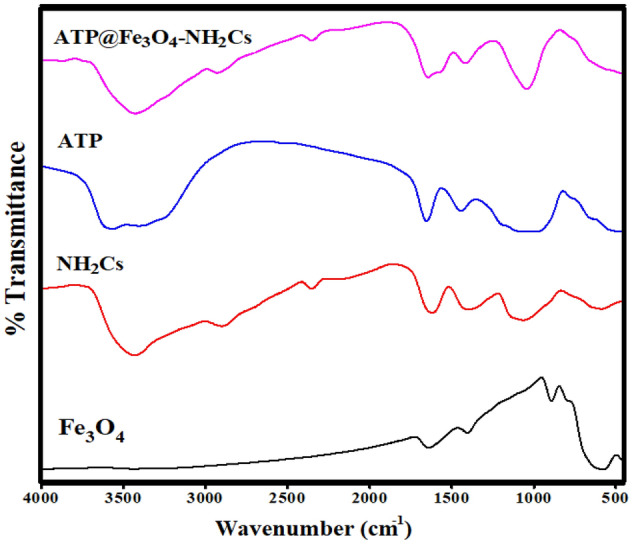
FTIR of Fe3O4, NH2Cs, ATP and ATP@Fe3O4-NH2Cs composite.
XPS
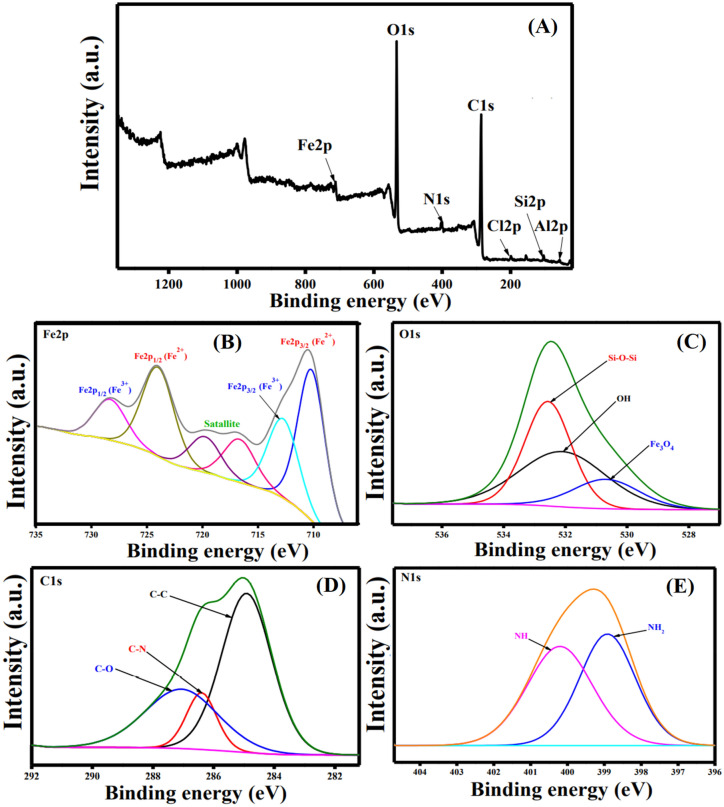
The elemental composition of the as-fabricated ATP@Fe3O4-NH2Cs composite was scrutinized by XPS analysis. The wide-spectrum (Fig. 3A) illustrates the main elements of the composite; Al2p, Si2p, Cl2p, C1s, N1s, O1s and Fe2p at binding energy (BE) of 58.08, 103.15, 198.79, 286.35, 401.47, 533.04 and 712.18 eV, respectively47.The high-resolution spectrum of Fe2p (Fig. 3B) verifies that the as-fabricated composite contains Fe2+ (Fe 2p3/2: 710.17 eV, Fe 2p1/2: 724 eV and satellites: 716.66 and 719.77 eV) and Fe3+ (Fe 2p3/2: 712.72 eV,Fe 2p1/2: 728.3 eV)48. Moreover, the high-resolution spectrum of O1s (Fig. 3C) shows a peak at BE of 530.63 eV which is ascribed to the oxygen atoms in Fe3O4 lattice. Furthermore, the peak at BE of 532.18 eV is related to OH groups, while the peak at BE of 532.59 is due to Si–O–Si of ATP clay49. Besides, the high-resolution of N1s (Fig. 3D) demonstrates the distinguishing peaks of NH and NH2 groups at BE of 400.26 and 398.97 eV, respectively. The high-resolution of C1s (Fig. 3E) reveals three peaks at BE of 287.06 (for C–O), 286.4 (for C–N) and 284.91 (for C–C), respectively50.
Figure 3.
XPS spectra (A) wide scan of ATP@Fe3O4-NH2Cs composite, (B) Fe2p, (C) O1s, (D) C1s and (E) N1s.
SEM
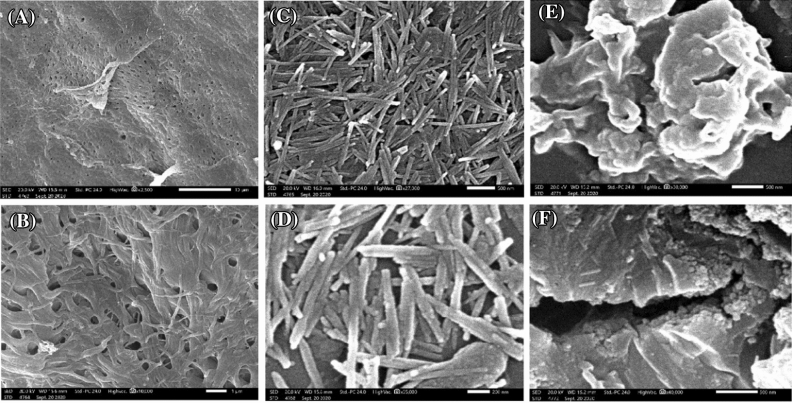
SEM images (Fig. 4A,B) depicted that NH2Cs surface looks like a spongy network with open and interconnected pores. While, SEM images (Fig. 4C,D) showed that ATP clay has a rod-like structure in nano size. On the other hand, SEM images (Fig. 4E,F) revealed the layered structure of ATP@Fe3O4-NH2Cs composite with some interlayer spaces. Furthermore, brightness spots in some regions was noticed confirming the existence of exfoliation of ATP particles in the NH2Cs matrix51.
Figure 4.
SEM of (A,B) NH2Cs, (C,D) ATP and (E,F) ATP@Fe3O4-NH2Cs composite.
TGA
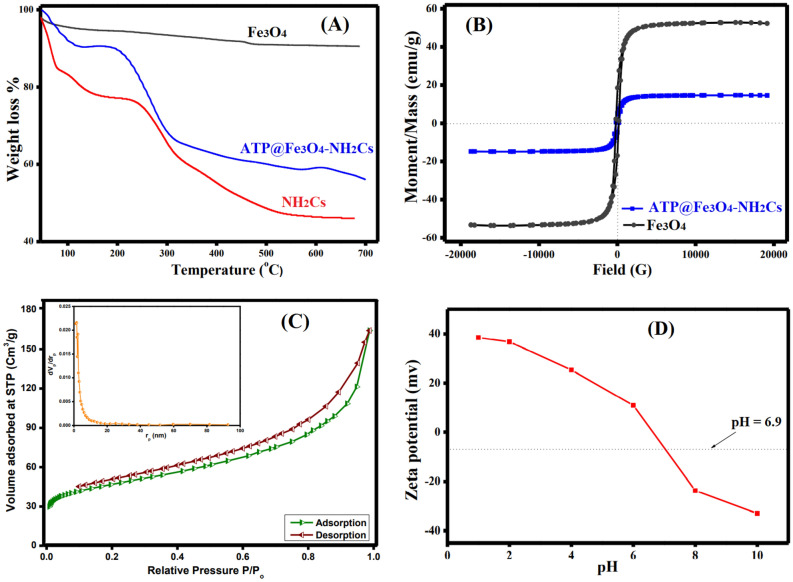
Thermal stability of the fabricated samples was scrutinized using TGA analysis at the temperature range from 45 to 700 °C (Fig. 5A). TGA curve of pure Fe3O4 illustrates a slight weight loss of about 5.75% from 45 to 450 °C which is ascribed to the removal of moisture52. Moreover, TGA curve of NH2Cs depicts three weight loss stages; the first stage was recorded up to 150 °C which may be attributed to the evaporation of the adsorbed water, while the second one was recorded up to 350 °C which may be due to the dehydration of the saccharide rings and depolymerization of NH2Cs53. Besides, the third weight loss that was recorded up to 600 °C elucidates the complete decomposition of NH2Cs54. It is apparent from TGA curve of ATP@Fe3O4-NH2Cs composite that the combination of NH2Cs with ATP and Fe3O4 ameliorates its thermal behavior at which the total weight loss of NH2Cs and ATP@Fe3O4-NH2Cs composite was 53.95 and 43.92%, respectively.
Figure 5.
(A) TGA of Fe3O4, NH2Cs and ATP@Fe3O4-NH2Cs composite, (B) VSM of Fe3O4 and ATP@Fe3O4-NH2Cs composite (C) N2 adsorption/desorption isotherm and pore size distribution and (D) ZB ATP@Fe3O4-NH2Cs composite.
VSM
Figure 5B represents the magnetic behaviors of the fabricated Fe3O4 and ATP@Fe3O4-NH2Cs composite. The magnetization loops of both Fe3O4 and ATP@Fe3O4-NH2Cs composite reveal a ferromagnetic behavior as the coericivity values were 198.76 and 90.68 G, respectively. Moreover, the saturation magnetization of Fe3O4and ATP@Fe3O4-NH2Cs composite were 52.31 and 14.53 emu/g, respectively. This expected decrease in the saturation magnetization of Fe3O4 may be due to the shielding of polymer–clay layer55,56. However, the declined saturation magnetization of ATP@Fe3O4–NH2Cs composite, itis sufficient enough to provide a perfect magnetic separation.
BET
Figure 5C depicts the N2 adsorption/desorption hysteresis loop and the pore size distribution of ATP@Fe3O4-NH2Cs composite. The hysteresis loop reveals microporous structure of ATP@Fe3O4-NH2Cs composite at which the relatively low pressure (P/Po < 0.05 atm) significantly increased. Besides, the BET isotherm represents type IV with H4 hysteresis loop, indicating the existence of mesoporous. Moreover, the specific surface area and the total pore diameter were 164.24 m2/g and was 1.50 nm.
Zeta potential
Figure 5D displays that the point of zero charges of ATP@Fe3O4-NH2Cs composite is 6.9. Thence at pH < 6.9; ATP@Fe3O4-NH2Cs surface is positively charged due to the protonation of NH2 groups, providing columbic interactions between the positive charges on the ATP@Fe3O4-NH2Cs composite surface and the negatively charged Cr(VI) ions. Contrariwise, beyond pH 6.9 ATP@Fe3O4-NH2Cs composite displays negative charges, causing electrostatic repulsion forces with the anionic Cr(VI).
Removal of Cr (VI) by ATP@Fe3O4-NH2Cs composite
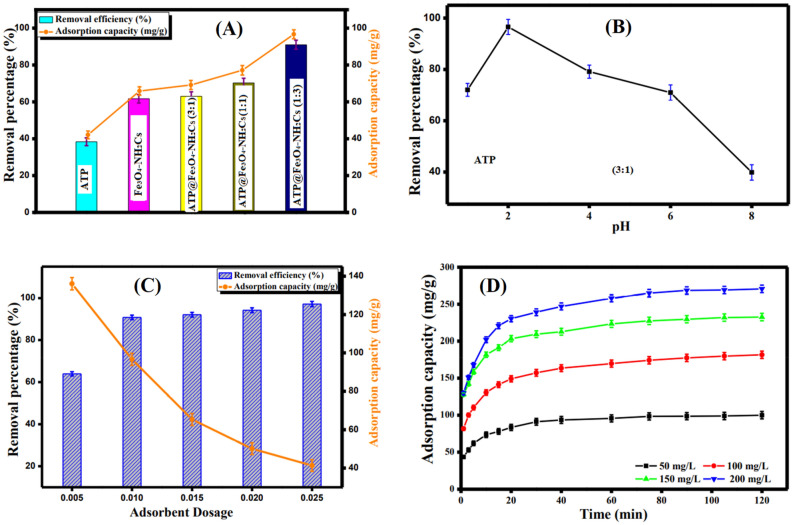
A comparative test was executed to compare the efficacy of the different synthesized composites to determine which ratio has the finest adsorption capacity towards the Cr(VI) ions under the same adsorption conditions. Moreover, the same test was conducted for the pristine materials to trace the improvement in their adsorption behaviors after combination. Figure 6A showed that the adsorption capacity value increased in the order of ATP@Fe3O4-NH2Cs(1:3) (96.68 mg/g) > ATP@Fe3O4-NH2Cs(1:1) (77.14 mg/g) > ATP@Fe3O4-NH2Cs(3:1) (69.16 mg/g) > Fe3O4-NH2Cs (65.88 mg/g) > ATP (42.1 mg/g). These results confirm the improvement in the adsorption characters of the three composites comparing with the pristine as a result of the generated synergetic effect between Fe3O4-NH2Cs and ATP clay. On the basis of these results, the synergetic effect of ATP was calculated and listed in Table 1. It was obvious that the incorporation of ATP clay greatly enhanced the removal efficiency of Fe3O4-NH2Cs. Moreover, the increase in the adsorption capacity with increasing the Fe3O4-NH2Cs dosage in the adsorption medium is most likely due to increasing the surface positive charges resultant from the extra amine groups of aminated chitosan (NH2Cs). Accordingly, ATP@Fe3O4-NH2Cs was picked out for the subsequent studies.
Figure 6.
(A) Adsorption profiles of Cr(VI) onto ATP, Fe3O4-NH2Cs, ATP@Fe3O4-NH2Cs (3:1), ATP@Fe3O4-NH2Cs (1:1) and ATP@Fe3O4-NH2Cs (1:3), (B) Effect of pH, (C) Effect of dosage and (D) Effect of initial Cr(VI) concentration on the adsorption capacity.
Table 1.
Synergetic effect of ATP clay to Fe3O4-NH2Cs in the removal of Cr(VI).
| Sample code | Sample composition ATP : Fe3O4-NH2Cs |
qcal (mg/g) |
qexp (mg/g) |
Synergetic effect (%) |
|---|---|---|---|---|
| ATP@Fe3O4-NH2Cs 1:3 | (1:3) | 59.94 | 96.68 | 61.30 |
| ATP@Fe3O4-NH2Cs 1:1 | (1:1) | 53.99 | 77.14 | 42.88 |
| ATP@Fe3O4-NH2Cs 3:1 | (3:1) | 48.05 | 69.16 | 44.00 |
Effect of pH
Indeed, pH dominates the form of Cr(VI) in the aqueous solution, where Cr(VI) presents as H2CrO4 at pH = 1, while it exists as Cr2O72− and HCrO4- at pH ranging from 2 to 6, however, CrO42− is the prime form at pH > 657. In general, at high acidic medium NH2 groups protonate to NH3+, that charges ATP@Fe3O4-NH2Cs composite surface with a positive charge. It is apparent from Fig. 6B that the increase in pH from 1 to 2 results in an increase in the removal percentage from 65.53 to 90.81% and the adsorption capacity from 72.03 to 96.54 mg/g. This anticipated behavior can be assigned to the existence of Cr(VI) at pH = 1 in a neutral form (H2CrO4) which leads to a decrease in the columbic interactions between the cationic groups of ATP@Fe3O4-NH2Cs composite and the neutral H2CrO4 molecules7. However, at pH 2, there are resilient electrostatic interactions between the protonated NH3+ positive groups on the adsorbent surface and the negative charges of Cr(VI) species. One the other hand, beyond pH 2 there is an anticipated decrease in the number of protonated amine groups on the ATP@Fe3O4-NH2Cs surface. Consequently, the electrostatic interactions between ATP@Fe3O4-NH2Cs composite and Cr(VI) decreases, so the removal percentage and the adsorption capacity directly dwindle from 90.81% and 96.54 mg/g to 38.57% and 39.85 mg/g, respectively58. Based on these results, pH 2 was selected as an optimum pH value for the following adsorption studies.
Effect of ATP@Fe3O4-NH2Cs dosage
Figure 6C denotes the effect of adsorbent dosage on the adsorption profile. It is evident that increasing the ATP@Fe3O4-NH2Cs composite dosage from 0.005 to 0.025 g leads directly to a dramatically reduction in the adsorbed quantity of Cr(VI) from 136.07 to 41.34 mg/g, respectively, which may be attributed to the aggregation of ATP@Fe3O4-NH2Cs particles. Contrariwise, the Cr(VI) removal % was gradually increased from 63.17 to 97.17% with increasing the composite dosage as a result of increasing the adsorption active sites on the composite surface59.
Effect of initial concentration
Figure 6D points out that the adsorption capacity value significantly increases from 99.99 to 270.68 mg/g with increasing Cr(VI) concentration from 50 to 200 mg/L. These findings are expected due to increasing the driving forces that overcomes the mass transfer resistance of Cr(VI) ions from bulk to the ATP@Fe3O4-NH2Cs surface with increasing the initial Cr(VI) concentration. On the contrary, Figure (S1) shows a decline in the removal (%) value from 94.24 to 64.93% with rising the Cr(VI) concentration, which could be explained by the shortage of the adsorption active sites at constant adsorbent dosage12.
Adsorption isotherms
To deduce the interaction sort between Cr(VI) and ATP@Fe3O4-NH2Cs composite, the obtained equilibrium data were scrutinized by bountiful isotherm models like Langmuir, Freundlich, Temkin and Dubinin-Radushkevich (D-R). The linearized isotherm equations are listed in Table S160,61.
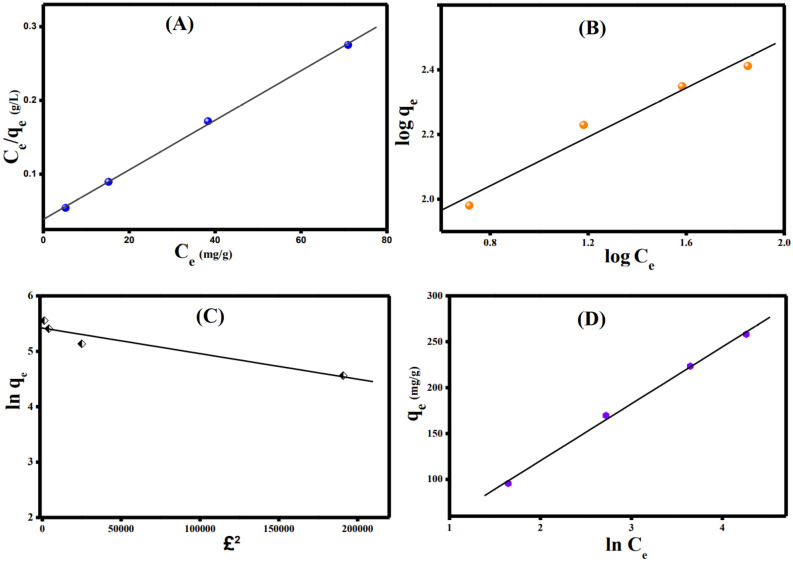
The plots of the applied isotherm models are illustrated in Fig. 7A–D. It was inferred from R2 values (Table 2) that the inspected Cr(VI) adsorption process obeys Langmuir (0.999) and Temkin (0.998) more than Freundlich (0.953) and D-R (0.915).
Figure 7.
Isotherms plots for the Cr (VI) adsorption onto ATP@Fe3O4-NH2Cs composite; (A) Langmiur, (B) Freundlich, (C) Temkin, and (E) D-R.
Table 2.
The parameters derived from isotherm models for the adsorption of Cr(VI) ions onto ATP@Fe3O4-NH2Cs composite.
| Isotherm model | Parameter | Value |
|---|---|---|
| Langmuir | qm (mg/g) | 294.118 |
| b (L/mg) | 0.088 | |
| R2 | 0.999 | |
| Freundlich | n | 2.646 |
| kF (mg/g)(mg/L)-1/n | 84.411 | |
| R2 | 0.953 | |
| Temkin | A (L/g) | 0.946 |
| B (J/mol) | 61.948 | |
| b (KJ/mol) | 0.039 | |
| R2 | 0.998 | |
| D-R | qs | 225.56 |
| Kad (mol2/K2J2) | 5 × 10–6 | |
| R2 | 0.915 | |
| E (kJmol-1) | 0.316 |
The computed Langmuir parameters clarify that qmax of Cr(VI) onto ATP@Fe3O4-NH2Cs composite is 294.12 mg/g, agreeing with the actual maximum adsorption capacity (270.68 mg/g). Moreover, Freundlich constant evinces the favorability of the Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs composite at which n > 2. In addition, Temkin infers that the Cr(VI) ions adsorb onto ATP@Fe3O4-NH2Cs composite via physical adsorption, agreeing with D-R model result since the calculated bonding energy ( < 8 kJmol-1. In general, physical adsorption takes place via weak Van der Waals interactions, so the Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs composite requires a low adsorption energy62.
Adsorption kinetics
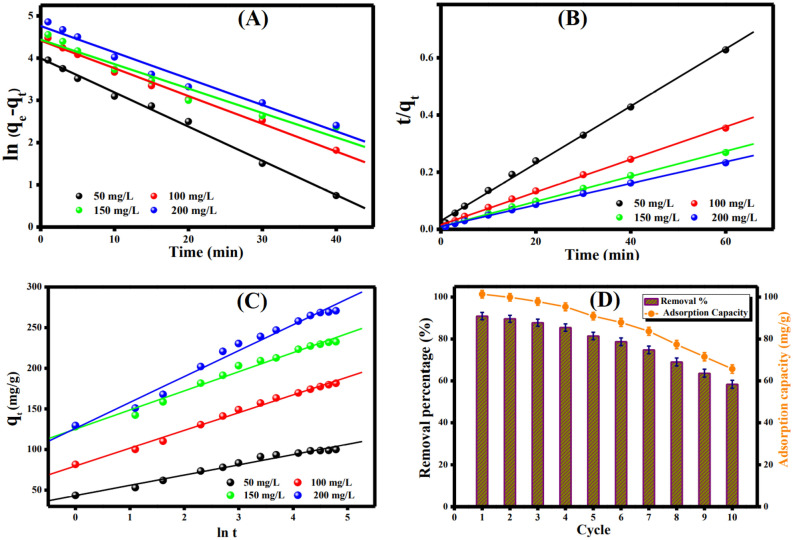
The Cr(VI) adsorption mechanism of onto ATP@Fe3O4-NH2Cs composite was identified utilizing Pseudo 1st order, Pseudo 2nd order and Elovich (Fig. 8A–C). The linearized kinetic equations were summarized in Table S2.
Figure 8.
Kinetic plots for the Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs composite; (A) Pseudo 1st order, (B) Pseudo 2nd order, and (C) Elovich and (D) Regeneration and reusability of ATP@Fe3O4-NH2Cs composite.
It was concluded that the Cr(VI) adsorption process onto ATP@Fe3O4-NH2Cs best fits pseudo 2nd order based on the R2 values (Table 3). Also, the computed q values from pseudo 2nd order seem to resemble the experimental values, evincing the suitability of pseudo 2nd order to represent the studied adsorption process. In addition, it was noticed a decline in the k2 values with the rising in the Cr(VI) initial concentration, suggesting the chemical adsorption process of Cr(VI) onto ATP@Fe3O4-NH2Cs composite59. Moreover, the computed Elovich coefficients elucidate that the rate of Cr(VI) adsorption is vaster than the desorption since the α values exceed the β values63.
Table 3.
Adsorption kinetic model parameters of the adsorption of Cr(VI) onto ATP@Fe3O4-NH2Cs composite.
| Kinetic models and parameters | Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | |||||
| qe, exp(mg/g) | 99.99 | 181.51 | 232.56 | 270.68 | ||||
| Pseudo 1storder | ||||||||
| qe,cal(mg/g) | 54.36 | 82.76 | 84.50 | 116.69 | ||||
| k1(min-1) | 0.081 | 0.066 | 0.058 | 0.062 | ||||
| R2 | 0.995 | 0.992 | 0.954 | 0.971 | ||||
| Pseudo 2ndorder | ||||||||
| qe,cal(mg/g) | 100 | 175.44 | 227.27 | 263.16 | ||||
| k2(g.mg-1.min-1) | 0.003 | 0.002 | 0.001 | 0.0009 | ||||
| R2 | 0.998 | 0.998 | 0.999 | 0.999 | ||||
| Elovich | ||||||||
| α (mg/g min) | 388.97 | 834.27 | 4783.07 | 1658.44 | ||||
| β (g/mg) | 0.0792 | 0.0457 | 0.425 | 0.0314 | ||||
| R2 | 0.979 | 0.993 | 0.985 | 0.983 | ||||
Thermodynamics
To assess the impact of change the reaction temperature on the nature of Cr(VI) adsorption process onto ATP@Fe3O4-NH2Cs composite, the thermodynamics parameter such as, change in entropy (ΔSº), change in enthalpy (ΔHº) and change in free energy (ΔGº) were reckoned from Eqs. 3 and 4.
| 3 |
| 4 |
where, is the thermodynamic equilibrium constant; Ce and CAe are the Cr(VI) concentration in the solution and onto ATP@Fe3O4-NH2Cs surface at equilibrium, respectively. R and T are gas constant and adsorption temperature, respectively.
The computed thermodynamics parameters demonstrate that the Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs is randomness and endothermic process owing to the positive values of both ∆So and ∆Ho that have been reckoned from Van't Hoff Plot (Figure S2). Also, ∆Ho value suggesting the chemical adsorption process since it falls between 40 and 200 kJ/mol64. Besides, the negative values of ∆Go (Table 4) elucidate the spontaneity of the Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs59.
Table 4.
Thermodynamic parameters of the adsorption of Cr(VI) onto ATP@Fe3O4-NH2Cs composite.
| ΔG°(kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) | |||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | 8.45 | 46.77 |
| − 13.98 | − 14.40 | − 14.86 | − 15.33 | ||
Reusability
To evaluate the ability of ATP@Fe3O4-NH2Cs magnetic composite to reuse for several adsorption cycles, ten successive adsorption–desorption processes were executed. Figure 8D points out that the developed adsorbent still retains respectable adsorption characteristics even after 10 cycles with maximum adsorption capacity of 62.54 mg/g and maximum Cr(VI) removal % of 58.36%, indicating the well-recycling property of the as-fabricated ATP@Fe3O4-NH2Cs magnetic composite. This fascinating behavior to the as-fabricated ATP@Fe3O4-NH2Cs composite is may be due to its magnetic property that provides a perfect separation to the composite after the adsorption process without losing in its mass.
Comparison with other studies
To assess the developing behavior of our novel adsorbent, a comparison study was executed between the as-fabricated ATP@Fe3O4-NH2Cs composite and other reported adsorbents in previous studies (Table 5). Notably, ATP@Fe3O4-NH2Cs composite has a dual function; an excellent adsorption capacity towards Cr(VI) ions (294.12 mg/g) and a fast adsorption process as it reaches equilibrium at about one hour. This fascinating adsorption property of ATP@Fe3O4-NH2Cs composite is most likely due to the synergistic effect between ATP and Fe3O4-NH2Cs. Over and above, the extra- amine groups boost the cationic nature of the composite surface, and consequently causing more electrostatic interactions between the anionic Cr(VI) and the positively charged surface of ATP@Fe3O4-NH2Cs composite.
Table 5.
Comparison of the maximum adsorption of Cr(VI) with numerous adsorbents.
| Adsorbent | qmax (mg/g) |
Equilibrium time (min) | References |
|---|---|---|---|
| Chitosan nanofibers | 131.58 | 480 | 70 |
| Malic acid-chitosan beads | 382.20 | 120 | 71 |
| MAC–attapulgite composite | 119.62 | 120 | 72 |
| Attapulgite-supported nZVI composite | 266.65 | 720 | 73 |
| Polypyrrole/molybdenum disulfide composite | 257.73 | 900 | 74 |
| bentonite@MnFe2O4composite | 161.30 | 90 | 75 |
| Polyaniline/Bi(III) iodomolybdate composite | 240.90 | 40 | 76 |
| BM-FeS2@BC700 | 134.00 | 100 | 77 |
| MoS2@LDC) composite | 198.70 | 40 | 78 |
| Fe3O4/ZIF-67@AmCs beads | 119.05 | 80 | 65 |
| ATP@Fe3O4-NH2Cs composite | 294.12 | 60 | This work |
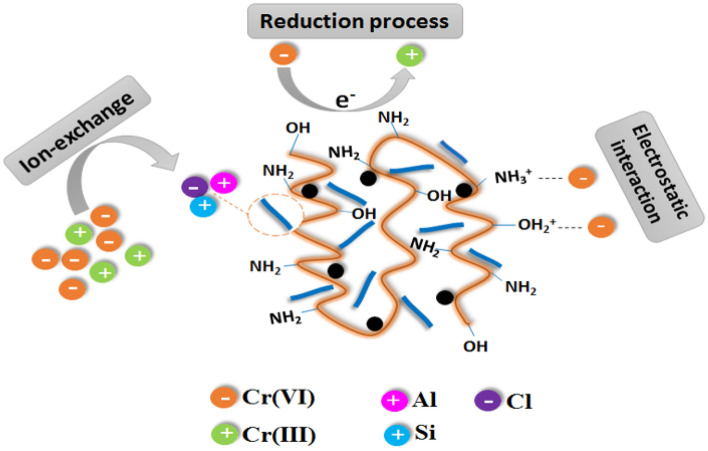
Adsorption mechanism
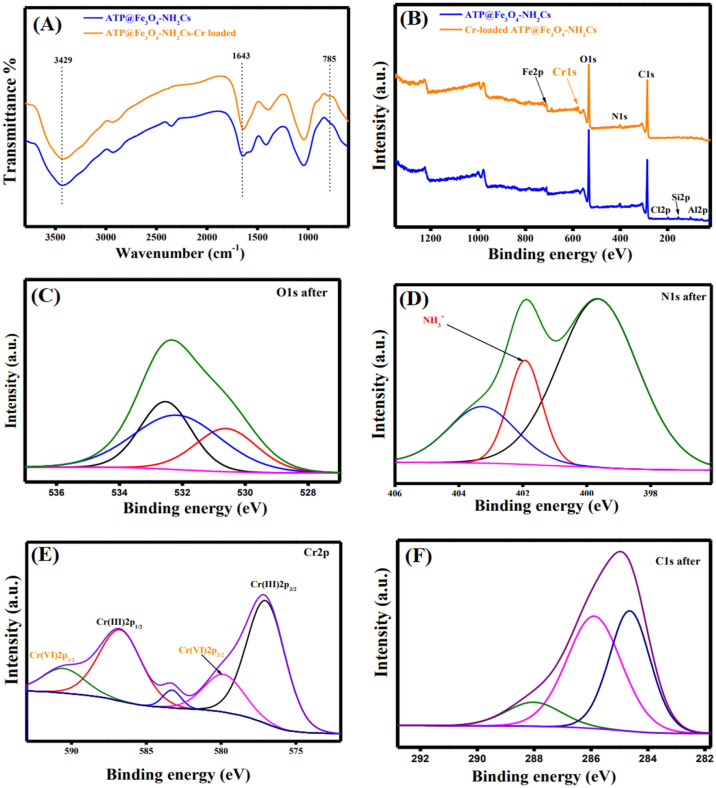
The mechanism of Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs adsorbent was deduced depending on the gained FTIR and XPS data before and after the adsorption process. FTIR spectrum of ATP@Fe3O4-NH2Cs composite after adsorption of Cr(VI) (Fig. 9A) illustrates the distinctive peak of tetrahedron CrO4 at 785 cm−165. Besides, the bands related to both of –NH2 and –OH− groups are moved from 1643 and 3429 cm−1 to 1639 and 3424 cm−1, respectively, implying that hydroxyl and amine groups of ATP@Fe3O4-NH2Cs composite are involved in the adsorption of Cr(VI) ions66. Moreover, XPS spectrum of ATP@Fe3O4-NH2Cs composite after the adsorption process (Fig. 9B) reveals the belonging peak to Cr2p at BE of 578.16 eV. In addition, the results of Fig. 9C approve that the adsorption process occurs via reduction of Cr(VI) to Cr(III) at which the characteristics peaks of Cr(III) 2p1/2 and Cr(III) 2p3/2 emerged at BE of 586.75 and 577.01 eV, respectively66. While, the peaks at BE of 592.98 and 579.82 eV are related to Cr(VI) 2p1/2 and Cr(VI) 2p3/2, respectively. Furthermore, there is a decline in the intensity of Al2p, Si2p and Cl2p peaks which may be interpreted by the partial exchange of attapulgite metal ions with Cr(VI) and Cr(III), suggesting the ion exchange mechanism47,67. On the other hand, Fig. 9D shows the peaks at BE of 398.38 and 400.16 eV which are ascribed to –NH2 and NH groups, respectively. Whereas, the spectrum of N1s after the adsorption of Cr(VI) infers the protonation of the amine group at low pH, since the distinctive peak of NH3+ appeared at BE of 402 eV, suggesting the possibility of electrostatic interaction mechanism between the anionic Cr(VI) ions and NH3+ on the surface of the composite. Furthermore, the wide-spectrum of O1s after the adsorption process (Fig. 9E) clearly revealed a decrease in the intensity of OH and Si–O–Si peaks which could be attributed to exchange of OH and Si with Cr(VI) and Cr(III) ions68. In addition, it was found a slight shift around 0.3 eV in the binding energy of C1s after the adsorption of Cr(VI) (Fig. 9F) which may be ascribed to the reaction of Cr(VI) ions with oxygen and nitrogen function groups, agreeing with FTIR results69. To sum, FTIR and XPS results suppose that mechanism of Cr(VI) adsorption onto ATP@Fe3O4-NH2Cs composite involve the electrostatic interactions, reduction of Cr(VI) to Cr(III) and ion-exchanging (Fig. 10).
Figure 9.
(A) FTIR of ATP@Fe3O4-NH2Cs composite before and after adsorption of Cr(VI), (B) XPS spectra of ATP@Fe3O4-NH2Cs before and after adsorption of Cr(VI), (C) Cr2p, (D) N1s, (E) O1s and (F) C1s after adsorption of Cr(VI) onto ATP@Fe3O4-NH2Cs composite.
Figure 10.
Proposed mechanism for removal Cr(VI) onto ATP@Fe3O4-NH2Cs composite.
Conclusion
In this study, ATP@Fe3O4-NH2Cs composite was formulated with different proportions for efficient adsorption for Cr(VI) ions from their aqueous solutions. The utilized characterization tools elucidated the good thermal and magnetic characteristics of the as-fabricated ATP@Fe3O4-NH2Cs composite in addition to its higher surface area. Furthermore, batch adsorption experiments clarified that the best adsorption capacity values were attained at pH 2 and achieved by ATP@Fe3O4-NH2Cs(1:3). Moreover, isotherms studies revealed an analogy between the calculated maximum adsorption capacity under Langmuir isotherm model (294.12 mg/g) and the experimental one (270.68 mg/g). In addition, kinetics studies validated that the adsorption process follows the pseudo 2nd order kinetic model, while the thermodynamic parameters recognized the process to be endothermic, spontaneous and randomness. Furthermore, the results assumed that the adsorption process of Cr(VI) ions occurred via the electrostatic interaction between opposite charges, reduction of Cr(VI) to Cr(III) and ion-exchanging mechanisms. Finally, reusability test proved also the excellent potential of ATP@Fe3O4-NH2Cs adsorbent composite to be reuse for several times, which is a beneficial for its application for removing of Cr(VI) ions from contaminated water. It can be concluded that the as-fabricated ATP@Fe3O4-NH2Cs composite could be applied as sustainable and reusable adsorbent for removing Cr(VI) ions from wastewater.
Supplementary Information
Author contributions
A.S.E. and A.M.O. proposed the research concept; E.M.A. conducted the experiments; A.S.E., A.M.O. and E.M.A. analyzed, interpreted the data and wrote the manuscript; A.S.E., A.M.O. M.S.M. and E.M.A. revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdelazeem S. Eltaweil, Email: abdelazeemeltaweil@alexu.edu.eg
Ahmed M. Omer, Email: amomar@srtacity.sci.eg
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96145-6.
References
- 1.El-Monaem EMA, El-Latif MMA, Eltaweil AS, El-Subruiti GM. Cobalt nanoparticles supported on reduced amine-functionalized graphene oxide for catalytic reduction of nitroanilines and organic dyes. NANO. 2021;21:50039. [Google Scholar]
- 2.Sallam S, El-Subruiti G, Eltaweil A. Facile synthesis of Ag–γ-Fe 2 O 3 superior nanocomposite for catalytic reduction of nitroaromatic compounds and catalytic degradation of methyl orange. Catal. Lett. 2018;148:3701–3714. doi: 10.1007/s10562-018-2569-z. [DOI] [Google Scholar]
- 3.Dubey S, Gusain D, Sharma YC. Kinetic and isotherm parameter determination for the removal of chromium from aqueous solutions by nanoalumina, a nanoadsorbent. J. Mol. Liq. 2016;219:1–8. doi: 10.1016/j.molliq.2016.01.021. [DOI] [Google Scholar]
- 4.Long Z, Zhang G, Du H, Zhu J, Li J. Preparation and application of BiOBr-Bi2S3 heterojunctions for efficient photocatalytic removal of Cr (VI) J. Hazard. Mater. 2021;407:124394. doi: 10.1016/j.jhazmat.2020.124394. [DOI] [PubMed] [Google Scholar]
- 5.Li N, et al. Efficient removal of chromium from water by Mn3O4@ ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B. 2017;214:126–136. doi: 10.1016/j.apcatb.2017.05.041. [DOI] [Google Scholar]
- 6.Khan TA, Nazir M, Ali I, Kumar A. Removal of chromium (VI) from aqueous solution using guar gum–nano zinc oxide biocomposite adsorbent. Arab. J. Chem. 2017;10:S2388–S2398. doi: 10.1016/j.arabjc.2013.08.019. [DOI] [Google Scholar]
- 7.Omer A, et al. Fabrication of tetraethylenepentamine functionalized alginate beads for adsorptive removal of Cr (VI) from aqueous solutions. Int. J. Biol. Macromol. 2019;125:1221–1231. doi: 10.1016/j.ijbiomac.2018.09.097. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed A, et al. Removal of chromium (VI) from aqueous solutions using surface modified composite nanofibers. J. Colloid Interface Sci. 2017;505:682–691. doi: 10.1016/j.jcis.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 9.Chen K-Y, et al. Removal and simultaneous reduction of Cr (VI) by organo-Fe (III) composites produced during coprecipitation and coagulation processes. J. Hazard. Mater. 2019;376:12–20. doi: 10.1016/j.jhazmat.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 10.Reyes-Serrano A, López-Alejo JE, Hernández-Cortázar MA, Elizalde I. Removing contaminants from tannery wastewater by chemical precipitation using CaO and Ca (OH) 2. Chin. J. Chem. Eng. 2020;2:2. [Google Scholar]
- 11.Eltaweil A, Mohamed HA, Abd El-Monaem EM, El-Subruiti G. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020;31:1253–1263. doi: 10.1016/j.apt.2020.01.005. [DOI] [Google Scholar]
- 12.Omer AM, Elgarhy GS, El-Subruiti GM, Khalifa RE, Eltaweil AS. Fabrication of novel iminodiacetic acid-functionalized carboxymethyl cellulose microbeads for efficient removal of cationic crystal violet dye from aqueous solutions. Int. J. Biol. Macromol. 2020;148:1072–1083. doi: 10.1016/j.ijbiomac.2020.01.182. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed K, Sahu O. Recovery of chromium from tannery industry waste water by membrane separation technology: Health and engineering aspects. Sci. Afr. 2019;4:e00096. [Google Scholar]
- 14.Zhao Z, Zhang B, Chen D, Guo Z, Peng Z. Simultaneous reduction of vanadium (V) and chromium (VI) in wastewater by nanosized ZnWO4 Photocatalysis. J. Nanosci. Nanotechnol. 2016;16:2847–2852. doi: 10.1166/jnn.2016.10766. [DOI] [PubMed] [Google Scholar]
- 15.Xing J, et al. Electrically switched ion exchange based on polypyrrole and carbon nanotube nanocomposite for the removal of chromium (VI) from aqueous solution. Ind. Eng. Chem. Res. 2018;57:768–774. doi: 10.1021/acs.iecr.7b03520. [DOI] [Google Scholar]
- 16.Wu X, et al. Cr (III) recovery in form of Na2CrO4 from aqueous solution using improved bipolar membrane electrodialysis. J. Membr. Sci. 2020;11:8097. [Google Scholar]
- 17.Tamer TM, et al. Formation of zinc oxide nanoparticles using alginate as a template for purification of wastewater. Environ. Nanotechnol. Monit. Manag. 2018;10:112–121. [Google Scholar]
- 18.Omer A, et al. Development of iron oxide nanoparticles using alginate hydrogel template for chromium (VI) ions removal. Desalin. Water Treat. 2020;175:229–243. doi: 10.5004/dwt.2020.24916. [DOI] [Google Scholar]
- 19.Wei J, et al. Carbon-coated montmorillonite nanocomposite for the removal of chromium (VI) from aqueous solutions. J. Hazard. Mater. 2019;368:541–549. doi: 10.1016/j.jhazmat.2019.01.080. [DOI] [PubMed] [Google Scholar]
- 20.Sahu S, Kar P, Bishoyi N, Mallik L, Patel RK. Synthesis of polypyrrole-modified layered double hydroxides for efficient removal of Cr (VI) J. Chem. Eng. Data. 2019;64:4357–4368. doi: 10.1021/acs.jced.9b00444. [DOI] [Google Scholar]
- 21.Sahu S, Pahi S, Sahu JK, Sahu UK, Patel RK. Kendu (Diospyros melanoxylon Roxb) fruit peel activated carbon—an efficient bioadsorbent for methylene blue dye: equilibrium, kinetic, and thermodynamic study. Environ. Sci. Pollut. Res. 2020;27:22579–22592. doi: 10.1007/s11356-020-08561-2. [DOI] [PubMed] [Google Scholar]
- 22.Tamer TM, et al. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: in vitro and in vivo evaluations. J. Biotechnol. 2020;310:103–113. doi: 10.1016/j.jbiotec.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Omer AM, Ahmed MS, El-Subruiti GM, Khalifa RE, Eltaweil AS. pH-sensitive alginate/carboxymethyl chitosan/aminated chitosan microcapsules for efficient encapsulation and delivery of diclofenac sodium. Pharmaceutics. 2021;13:338. doi: 10.3390/pharmaceutics13030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuwei C, Jianlong W. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu (II) removal. Chem. Eng. J. 2011;168:286–292. doi: 10.1016/j.cej.2011.01.006. [DOI] [Google Scholar]
- 25.Islam MN, Khan MN, Mallik AK, Rahman MM. Preparation of bio-inspired trimethoxysilyl group terminated poly (1-vinylimidazole)-modified-chitosan composite for adsorption of chromium (VI) ions. J. Hazard. Mater. 2019;379:120792. doi: 10.1016/j.jhazmat.2019.120792. [DOI] [PubMed] [Google Scholar]
- 26.El-Sayed E, Tamer TM, Omer AM, Mohy Eldin MS. Development of novel chitosan schiff base derivatives for cationic dye removal: methyl orange model. Desal. Water Treat. 2016;57:22632–22645. doi: 10.1080/19443994.2015.1136694. [DOI] [Google Scholar]
- 27.Purwanto M, et al. Biopolymer-based electrolyte membranes from chitosan incorporated with montmorillonite-crosslinked GPTMS for direct methanol fuel cells. RSC Adv. 2016;6:2314–2322. doi: 10.1039/C5RA22420A. [DOI] [Google Scholar]
- 28.Kyzas GZ, Bikiaris DN. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs. 2015;13:312–337. doi: 10.3390/md13010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuvaraja G, et al. Preparation of novel aminated chitosan schiff’s base derivative for the removal of methyl orange dye from aqueous environment and its biological applications. Int. J. Biol. Macromol. 2020;146:1100–1110. doi: 10.1016/j.ijbiomac.2019.09.236. [DOI] [PubMed] [Google Scholar]
- 30.Shebl A, Omer A, Tamer T. Adsorption of cationic dye using novel O-amine functionalized chitosan Schiff base derivatives: Isotherm and kinetic studies. Desalin. Water Treat. 2018;130:132–141. doi: 10.5004/dwt.2018.22986. [DOI] [Google Scholar]
- 31.Asiabi M, Mehdinia A, Jabbari A. Electrospun biocompatible Chitosan/MIL-101 (Fe) composite nanofibers for solid-phase extraction of Δ9-tetrahydrocannabinol in whole blood samples using Box-Behnken experimental design. J. Chromatogr. A. 2017;1479:71–80. doi: 10.1016/j.chroma.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Hou H, Zhou R, Wu P, Wu L. Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem. Eng. J. 2012;211:336–342. doi: 10.1016/j.cej.2012.09.100. [DOI] [Google Scholar]
- 33.Xu W, Chen Y, Zhang W, Li B. Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv. Powder Technol. 2019;30:493–501. doi: 10.1016/j.apt.2018.11.028. [DOI] [Google Scholar]
- 34.Salahuddin NA, EL-Daly HA, El Sharkawy RG, Nasr BT. Nano-hybrid based on polypyrrole/chitosan/grapheneoxide magnetite decoration for dual function in water remediation and its application to form fashionable colored product. Adv. Powder Technol. 2020;31:1587–1596. doi: 10.1016/j.apt.2020.01.030. [DOI] [Google Scholar]
- 35.Eltaweil AS, El-Tawil AM, Abd El-Monaem EM, El-Subruiti GM. Zero valent iron nanoparticle-loaded nanobentonite intercalated carboxymethyl chitosan for efficient removal of both anionic and cationic dyes. ACS Omega. 2021;6:6348–6360. doi: 10.1021/acsomega.0c06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Wang Q, Wang A. Synthesis and characterization of chitosan-g-poly (acrylic acid)/attapulgite superabsorbent composites. Carbohyd. Polym. 2007;68:367–374. doi: 10.1016/j.carbpol.2006.11.018. [DOI] [Google Scholar]
- 37.Liu Y, Liu P, Su Z, Li F, Wen F. Attapulgite–Fe3O4 magnetic nanoparticles via co-precipitation technique. Appl. Surf. Sci. 2008;255:2020–2025. doi: 10.1016/j.apsusc.2008.06.193. [DOI] [Google Scholar]
- 38.Chen H, Zhao J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption. 2009;15:381–389. doi: 10.1007/s10450-009-9155-z. [DOI] [Google Scholar]
- 39.Elbassyoni S, Kamoun EA, Taha TH, Rashed MA, ElNozahi FA. Effect of Egyptian attapulgite clay on the properties of PVA-HES–clay nanocomposite hydrogel membranes for wound dressing applications. Arab. J. Sci. Eng. 2020;2:1–13. [Google Scholar]
- 40.Duan Z, et al. Novel application of attapulgite on high performance and low-cost humidity sensors. Sensors Actuators B Chem. 2020;305:127534. doi: 10.1016/j.snb.2019.127534. [DOI] [Google Scholar]
- 41.El Bestawy E, El-Shatby BF, Eltaweil AS. Integration between bacterial consortium and magnetite (Fe 3 O 4) nanoparticles for the treatment of oily industrial wastewater. World J. Microbiol. Biotechnol. 2020;36:1–16. doi: 10.1007/s11274-020-02915-1. [DOI] [PubMed] [Google Scholar]
- 42.Liang XX, et al. Efficient adsorption of diclofenac sodium from aqueous solutions using magnetic amine-functionalized chitosan. Chemosphere. 2019;217:270–278. doi: 10.1016/j.chemosphere.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Eltaweil AS, Elshishini HM, Ghatass ZF, Elsubruiti GM. Ultra-high adsorption capacity and selective removal of Congo red over aminated graphene oxide modified Mn-doped UiO-66 MOF. Powder Technol. 2021;379:407–416. doi: 10.1016/j.powtec.2020.10.084. [DOI] [Google Scholar]
- 44.El-Subruiti G, Eltaweil A, Sallam S. Synthesis of active MFe2O4/γ-Fe2O3 nanocomposites (metal= Ni or Co) for reduction of nitro-containing pollutants and methyl orange degradation. NANO. 2019;14:1950125. doi: 10.1142/S179329201950125X. [DOI] [Google Scholar]
- 45.Quy DV, et al. Synthesis of silica-coated magnetic nanoparticles and application in the detection of pathogenic viruses. J. Nanomater. 2013;20:13. [Google Scholar]
- 46.Yousefian M, Rafiee Z. Cu-metal-organic framework supported on chitosan for efficient condensation of aromatic aldehydes and malononitrile. Carbohydr. Polym. 2020;228:115393. doi: 10.1016/j.carbpol.2019.115393. [DOI] [PubMed] [Google Scholar]
- 47.Hu S, et al. Preparation and application of alginate-Ca/attapulgite clay core/shell particle for the removal of uranium from aqueous solution. J. Radioanal. Nucl. Chem. 2017;314:307–319. doi: 10.1007/s10967-017-5427-3. [DOI] [Google Scholar]
- 48.Tang XQ, et al. Fe3O4 and metal–organic framework MIL-101 (Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta. 2018;179:43–50. doi: 10.1016/j.talanta.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 49.Han S, Yu H, Yang T, Wang S, Wang X. Magnetic activated-ATP@ Fe 3 O 4 nanocomposite as an efficient Fenton-like heterogeneous catalyst for degradation of ethidium bromide. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao W, Zhong D, Xu Y, Luo H, Zeng S. Nano zero-valent iron supported by macroporous styrene ion exchange resin for enhanced Cr (VI) removal from aqueous solution. J. Dispers. Sci. Technol. 2020;2:1–11. doi: 10.1080/01932691.2020.1848583. [DOI] [Google Scholar]
- 51.Inayatullah KS. Morphological, thermal, mechanical and solvent uptake of clay/nylon 6, 6 composites. J. Chilean Chem. Society. 2017;62:3562–3565. doi: 10.4067/s0717-97072017000303562. [DOI] [Google Scholar]
- 52.Omer AM, et al. Formulation of quaternized aminated chitosan nanoparticles for efficient encapsulation and slow release of curcumin. Molecules. 2021;26:449. doi: 10.3390/molecules26020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arsalani N, Fattahi H, Nazarpoor M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym. Lett. 2010;4:329–338. doi: 10.3144/expresspolymlett.2010.42. [DOI] [Google Scholar]
- 54.Nanaki S, et al. Thiolated chitosan masked polymeric microspheres with incorporated mesocellular silica foam (MCF) for intranasal delivery of paliperidone. Polymers. 2017;9:617. doi: 10.3390/polym9110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roveimiab Z, Mahdavian AR, Biazar E, Heidari KS. Preparation of magnetic chitosan nanocomposite particles and their susceptibility for cellular separation applications. J. Colloid Sci. Biotechnol. 2012;1:82–88. doi: 10.1166/jcsb.2012.1007. [DOI] [Google Scholar]
- 56.Fan L, Luo C, Sun M, Li X, Qiu H. Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surf., B. 2013;103:523–529. doi: 10.1016/j.colsurfb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Dehghani MH, Sanaei D, Ali I, Bhatnagar A. Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J. Mol. Liq. 2016;215:671–679. doi: 10.1016/j.molliq.2015.12.057. [DOI] [Google Scholar]
- 58.Parlayıcı Ş, Pehlivan E. Removal of chromium (VI) from aqueous solution using chitosan doped with carbon nanotubes. Materials Today: Proc. 2019;18:1978–1985. [Google Scholar]
- 59.Eltaweil AS, et al. Efficient removal of toxic methylene blue (MB) dye from aqueous solution using a metal-organic framework (MOF) MIL-101 (Fe): Isotherms, kinetics, and thermodynamic studies. Desalin. Water Treat. 2020;189:395–407. doi: 10.5004/dwt.2020.25599. [DOI] [Google Scholar]
- 60.Sahu S, Bishoyi N, Sahu MK, Patel RK. Investigating the selectivity and interference behavior for detoxification of Cr (VI) using lanthanum phosphate polyaniline nanocomposite via adsorption-reduction mechanism. Chemosphere. 2021;278:130507. doi: 10.1016/j.chemosphere.2021.130507. [DOI] [PubMed] [Google Scholar]
- 61.Sahu S, Bishoyi N, Patel RK. Cerium phosphate polypyrrole flower like nanocomposite: A recyclable adsorbent for removal of Cr (VI) by adsorption combined with in-situ chemical reduction. J. Ind. Eng. Chem. 2021;99:55–67. doi: 10.1016/j.jiec.2021.03.041. [DOI] [Google Scholar]
- 62.Araújo CS, et al. Elucidation of mechanism involved in adsorption of Pb (II) onto lobeira fruit (Solanum lycocarpum) using Langmuir Freundlich and Temkin isotherms. Microchem. J. 2018;137:348–354. doi: 10.1016/j.microc.2017.11.009. [DOI] [Google Scholar]
- 63.Ahamad K, Singh R, Baruah I, Choudhury H, Sharma M. Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder. Groundw. Sustain. Dev. 2018;7:452–458. doi: 10.1016/j.gsd.2018.06.005. [DOI] [Google Scholar]
- 64.Eltaweil AS, Abd El-Monaem EM, El-Subruiti GM, Abd El-Latif MM, Omer AM. Fabrication of UiO-66/MIL-101 (Fe) binary MOF/carboxylated-GO composite for adsorptive removal of methylene blue dye from aqueous solutions. RSC Adv. 2020;10:19008–19019. doi: 10.1039/D0RA02424D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Omer AM, Abd El-Monaem EM, Abd El-Latif MM, El-Subruiti GM, Eltaweil AS. Facile fabrication of novel magnetic ZIF-67 MOF@ aminated chitosan composite beads for the adsorptive removal of Cr (VI) from aqueous solutions. Carbohydr. Polym. 2021;265:118084. doi: 10.1016/j.carbpol.2021.118084. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Pan Z, Wang Y. Enhanced adsorption of cationic Pb (II) and anionic Cr (VI) ions in aqueous solution by amino-modified nano-sized illite-smectite clay. Environ. Sci. Pollut. Res. 2019;26:11126–11139. doi: 10.1007/s11356-019-04447-0. [DOI] [PubMed] [Google Scholar]
- 67.Vo AT, et al. Efficient removal of Cr (VI) from water by biochar and activated carbon prepared through hydrothermal carbonization and pyrolysis: adsorption-coupled reduction mechanism. Water. 2019;11:1164. doi: 10.3390/w11061164. [DOI] [Google Scholar]
- 68.Cao C-Y, et al. Low-cost synthesis of flowerlike α-Fe2O3 nanostructures for heavy metal ion removal: adsorption property and mechanism. Langmuir. 2012;28:4573–4579. doi: 10.1021/la300097y. [DOI] [PubMed] [Google Scholar]
- 69.Sivaranjini B, Mangaiyarkarasi R, Ganesh V, Umadevi S. Vertical alignment of liquid crystals over a functionalized flexible substrate. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-27039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Li Y, Cao L, Yang C. Enhanced chromium (VI) adsorption using nanosized chitosan fibers tailored by electrospinning. Carbohyd. Polym. 2015;125:206–213. doi: 10.1016/j.carbpol.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, et al. Malic acid-enhanced chitosan hydrogel beads (mCHBs) for the removal of Cr (VI) and Cu (II) from aqueous solution. Chem. Eng. J. 2018;353:225–236. doi: 10.1016/j.cej.2018.06.143. [DOI] [Google Scholar]
- 72.Hlungwane L, Viljoen EL, Pakade VE. Macadamia nutshells-derived activated carbon and attapulgite clay combination for synergistic removal of Cr (VI) and Cr (III) Adsorpt. Sci. Technol. 2018;36:713–731. doi: 10.1177/0263617417719552. [DOI] [Google Scholar]
- 73.Zhang W, et al. Effective removal of Cr (VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization. Chemosphere. 2019;221:683–692. doi: 10.1016/j.chemosphere.2019.01.070. [DOI] [PubMed] [Google Scholar]
- 74.Xiang L, et al. Polypyrrole coated molybdenum disulfide composites as adsorbent for enhanced removal of Cr (VI) in aqueous solutions by adsorption combined with reduction. Chem. Eng. J. 2020;2:127281. [Google Scholar]
- 75.Ahmadi A, Foroutan R, Esmaeili H, Tamjidi S. The role of bentonite clay and bentonite clay@ MnFe2O4 composite and their physico-chemical properties on the removal of Cr (III) and Cr (VI) from aqueous media. Environ. Sci. Pollut. Res. 2020;2:1–14. doi: 10.1007/s11356-020-07756-x. [DOI] [PubMed] [Google Scholar]
- 76.Kohila N, Subramaniam P. Removal of Cr (VI) using polyaniline based Sn (IV), Ce (IV) and Bi (III) iodomolybdate hybrid ion exchangers: Mechanistic and comparative study. J. Environ. Chem. Eng. 2020;8:104376. doi: 10.1016/j.jece.2020.104376. [DOI] [Google Scholar]
- 77.Tang J, Zhao B, Lyu H, Li D. Development of a novel pyrite/biochar composite (BM-FeS2@ BC) by ball milling for aqueous Cr (VI) removal and its mechanisms. J. Hazard. Mater. 2021;413:125415. doi: 10.1016/j.jhazmat.2021.125415. [DOI] [PubMed] [Google Scholar]
- 78.Chen H, et al. Constructing MoS2/Lignin-derived carbon nanocomposites for highly efficient removal of Cr (VI) from aqueous environment. J. Hazard. Mater. 2021;408:124847. doi: 10.1016/j.jhazmat.2020.124847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.