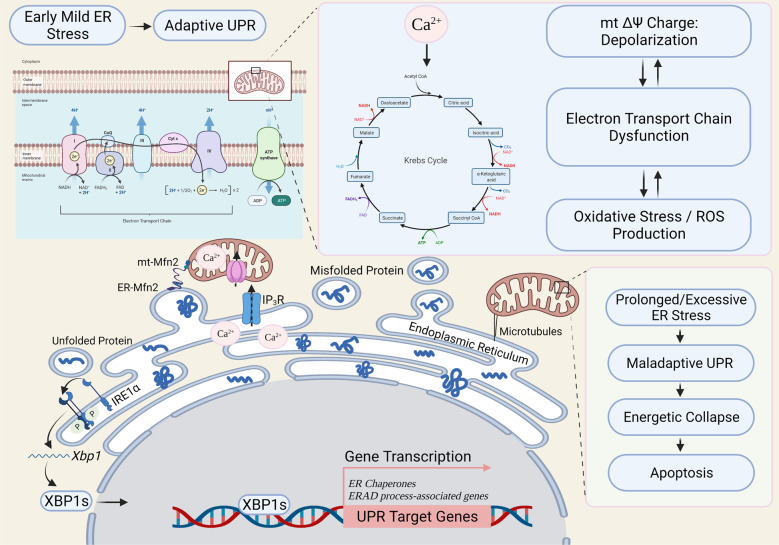
Fig. 4. Proposed working model of communication between mitochondria and endoplasmic reticulum stress.
Endoplasmic reticulum (ER) stress triggers an increase in mitochondrial metabolism, which mainly relies on organelle coupling and Ca2+ transfer. The onset of ER stress is accompanied with redistribution of reticular and mitochondrial networks towards the perinuclear region and a microtubule-dependent increase in connection. Physical interaction is mainly achieved by anchoring proteins, such as Mitofusin 2 (Mfn2), which allows buffering of intracellular Ca2+ from ER to mitochondria through its endoplasmic reticulum–mitochondria tethering activity, enhancing mitochondrial bioenergetics and ATP production consequently. The unfolded protein response (UPR) is a cellular self-defense adaptive mechanism to restore ER homeostasis. Crosstalk between the UPR pathways could facilitate a coordinated response to conditions of ER stress. During early mild ER stress, activated IRE1α then removes a 26-base intron from Xbp1 mRNA to generate a potent transcription factor XBP1s (Xbp1 spliced) that translocates into the nucleus and regulates a diverse array of genes, such as ER folding chaperones and ER-associated degradation (ERAD) process-associated genes. However, prolonged or excessive ER stress (e.g., induced by aging) would cause mitochondrial collapse and apoptotic cell death. ER: endoplasmic reticulum; UPR: unfolded protein response; ROS: reactive oxygen species; Mfn2: mitofusin 2; mt: mitochondrial; IRE1α: inositol-requiring kinase 1α (ER stress sensor); XBP1: X-box-binding protein 1; XBP1s: spliced form of XBP1; Krebs cycle: also known as TCA cycle (tricarboxylic acid cycle); IP3R: inositol trisphosphate receptor (Ca2+ channels); mt ΔΨ: mitochondrial membrane potential; ERAD: ER-associated degradation; ATP: adenosine 5´-triphosphate [74, 116,117,134, 142–145]. Created with BioRender.com.