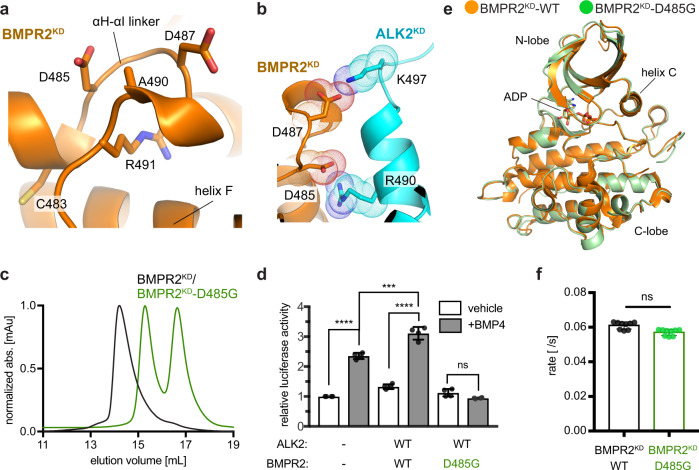
Fig. 6. Several PAH mutations map to the C1 dimer interface in the BMPR2 kinase and block its dimerization with the ALK2 kinase.
a Zoomed in view of the αH-αI linker within the C1 dimer interface in the BMPR2 kinase depicting residues C483, D485, D487, A490, and R491W mutated in PAH patients. b Zoomed in view depicting interactions made by D485 and D487 in BMPR2 with basic side chains of the residues at the C1 dimer interface in the ALK2 kinase. c Size-exclusion chromatograms of the ALK2KD/BMPR2KD-D485G complex (green trace) overlaid on the wild-type ALK2KD/BMPR2KD complex (black trace). d Effect of the D485G mutation on the BMP4-mediated activation of SMAD-dependent transcription by the BMPR2/ALK2 receptor complex, measured as described in Fig. 5c. Experiments were repeated at least three times and in each experiment each condition was quadruplicated with four independent biological samples. Means and standard deviation of the means (SDs) are indicated. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant by ANOVA with two-sided post hoc Tukey’s test. e A crystal structure of the BMPR2KD-D485G kinase, shown in green overlaid on the crystal structure of the wild-type BMPR2 kinase domain shown in orange (PDB: 3G2F). f In vitro measurement of the kinase activity of the wild-type BMPR2KD and BMPR2KD-D485G constructs. The rates measured at 2 μM enzyme concentration for each construct are shown. Each kinase assay measurement consists of three independent runs each time of samples in triplicates performed on samples obtained from separate purifications. Data shown are the means and standard deviation of the means (SDs) and was calculated using two-sided t-test, with no adjustments for multiple comparisons (ns, not significant, P > 0.05).