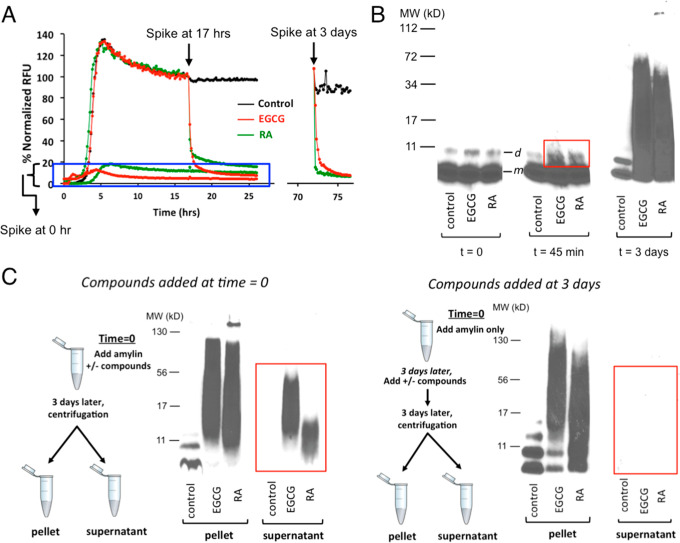
Figure 5.
Amylin amyloid remodeling induced by RA as probed by ThT fluorescence-based assays and orthogonal gel-based assays. (A) ThT fluorescence-based assay shows amylin amyloid remodeling after spiking of buffer control, RA, and EGCG before amylin amyloid is formed (t = 0 h), after amyloid has been formed (t = 17 h) or mature fibrils are formed (t = 3 days). (B) Single-fraction analysis of inhibitor-induced amyloid remodeling. Incubation of amylin amyloid with buffer control, EGCG, and RA leads to broad molecular weight range, SDS-resistant remodeled protein aggregates. Samples were separated by SDS-PAGE and probed by an antibody specific for human amylin (T-4157). The urea-dissolved, broadly distributed molecular weight of amylin-inhibitor aggregates were observed, while only monomer (indicated as “m”) and dimer bands (indicated as “d”) of amylin were seen in untreated samples. While with the quenching with denaturing conditions (6.5 M urea), both EGCG and RA induced broad molecular weight aggregates. SDS-resistant aggregates may be captured starting from 45 min of incubation (boxed in the red rectangle). (C) “Two-fractions” experiments demonstrated that EGCG and RA interact with monomeric amylin to form both soluble and insoluble aggregates of a broad molecular weight range, but such interactions with preformed fibrils lead to only insoluble aggregates. The experimental settings were the same as those described in panel B, except that the pellet and the supernatant fractions (two fractions) of each sample were separated by ultracentrifugation before the samples were separated by SDS-PAGE and probed by an antibody specific for human amylin (T-4157). Spiking of buffer control, EGCG, and RA was initiated either at 0 h (left panel) or after mature fibrils were formed (t = 3 days; right panel) as depicted in the schemes drawn on the left of each panel.