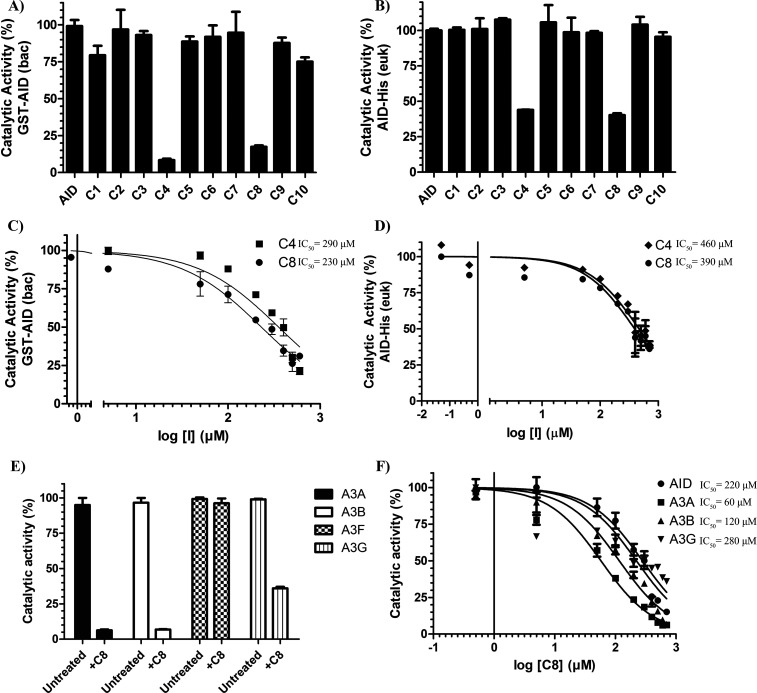
Figure 2.
First-generation inhibitor candidates inhibit purified AID and AID in whole cell extracts. (A) Catalytic activity of bacterially expressed and purified GST-AID treated with C1–C10 (n = 6 independent experiments conducted with three independently purified preparations of GST-AID). (B) Catalytic activity of eukaryotic-expressed AID in whole 293T cell lysate treated with C1–C10 (n = 3 independently prepared AID-expressing whole cell extracts). (C) Catalytic activity of GST-AID on C4 and C8 as a function of log inhibitor concentration. (D) Catalytic activity of AID-His 293T lysate as a function of log inhibitor concentration. (E) Catalytic activity of eukaryotic-expressed and purified GST-A3A, GST-A3B, GST-A3F, and GST-A3G treated with 700 μM C8. (F) Catalytic activity of GST-A3A, GST-A3B, and GST-A3G in comparison to bacterially expressed and purified GST-AID across a concentration range of C8. All experiments contained a negative control vehicle-only (140 mM DMSO) reaction which was designated as 100% AID activity. All AID reactions were performed at 37 °C for 2–4 h at pH 7.2 using 2 nM of the standard bubble oligonucleotide substrate TGCbub7 which has previously been demonstrated to be AID’s most favored substrate in the alkaline cleavage assay. GST-A3A, GST-A3B, GST-A3F, and GST-A3G reactions were incubated at 37 °C for 2 h in pH 6.0 using 2 nM of standard single-stranded oligonucleotide substrates containing a single target TTCA motif for A3A, A3B, and A3F and a single target CCC motif for A3G.