Abstract
Background
Vascular calcification is a closely linked to cardiovascular diseases, such as atherosclerosis, chronic kidney disease, diabetes, hypertension and aging. The extent of vascular calcification is closely correlate with adverse clinical events and cardiovascular all-cause mortality. The role of autophagy in vascular calcification is complex with many mechanistic unknowns.
Methods
In this review, we analyze the current known mechanisms of autophagy in vascular calcification and discuss the theoretical advantages of targeting autophagy as an intervention against vascular calcification.
Results
Here we summarize the functional link between vascular calcification and autophagy in both animal models of and human cardiovascular disease. Firstly, autophagy can reduce calcification by inhibiting the osteogenic differentiation of VSMCs related to ANCR, ERα, β-catenin, HIF-1a/PDK4, p62, miR-30b, BECN1, mTOR, SOX9, GHSR/ERK, and AMPK signaling. Conversely, autophagy can induce osteoblast differentiation and calcification as mediated by CREB, degradation of elastin, and lncRNA H19 and DUSP5 mediated ERK signaling. Secondly, autophagy also links apoptosis and vascular calcification through AMPK/mTOR/ULK1, Wnt/β-catenin and GAS6/AXL synthesis, as apoptotic cells become the nidus for calcium-phosphate crystal deposition. The failure of mitophagy can activate Drp1, BNIP3, and NR4A1/DNA‑PKcs/p53 mediated intrinsic apoptotic pathways, which have been closely linked to the formation of vascular calcification. Additionally, autophagy also plays a role in osteogenesis by regulating vascular calcification, which in turn regulates expression of proteins related to bone development, such as osteocalcin, osteonectin, etc. and regulated by mTOR, EphrinB2 and RhoA. Furthermore, autophagy also promotes vitamin K2-induced MC3T3 E1 osteoblast differentiation and FGFR4/FGF18- and JNK/complex VPS34–beclin-1-related bone mineralization via vascular calcification.
Conclusion
The interaction between autophagy and vascular calcification are complicated, with their interaction affected by the disease process, anatomical location, and the surrounding microenvironment. Autophagy activation in existent cellular damage is considered protective, while defective autophagy in normal cells result in apoptotic activation. Identifying and maintaining cells at the delicate line between these two states may hold the key to reducing vascular calcification, in which autophagy associated clinical strategy could be developed.
Keywords: Vascular calcification, Autophagy/mitophagy, Osteoblastic differentiation of VSMCs, Osteogenesis, AMPK/mTOR, HIF-1a/PDK4, EphrinB2, GAS6/AXL
Introduction
Regarded as a common pathological manifestation of patients with atherosclerosis, chronic kidney disease (CKD), diabetes, hypertension, postmenopausal syndrome, aortic stenosis [1–3] and the aging population [4], vascular calcification (VC) significantly correlated with cardiovascular and all-cause mortality, via deleterious mechanical effects on vascular compliance and vasomotion [5, 6]. Pathological abnormalities of VC may cause further adverse cardiovascular events and even induce death. The importance of VC to human health has attracted more attention, but the molecular mechanism of VC is under further investigation.
As a fundamental process for degradation and recycling of exhausted cellular components prevalent in eukaryotes, autophagy has recently been recognized in various physiological and pathological events, which presents a unique mechanism of self-regulating/cleaning [7–9]. Briefly, as a survival mechanism within an intracellular degradation system, autophagy process is composed of numerous chronological steps including sequestration, transport to lysosomes, degradation of cytoplasmic components, and utilization of degradation products. This self-degradative process tightly associates with both physiological and pathological status within normal embryonic and postnatal development in the behaviors of microautophagy, macroautophagy, chaperone-mediated autophagy and other new discovered manners [10].
The autophagic mechanism tightly associates with critical signaling pathways including PI3K/AKT, MAPK/Erk1/2, mTOR, AMPK, p53, HIF-1α/PDK4, β-catenin, ULK and Atg involved in regulation of the cellular autophagy [11, 12]. It has been intensely understood that autophagy functions in the cardiovascular diseases [13, 14], and the autophagic phenotype associated to vascular smooth muscle linking to steogenic differentiation [15], apoptosis [16], inflammation [17], Fibroblast Growth Factor 23 (FGF23)-Klotho [17, 18], Matrix Vesicle (MV) release [19], and oxidative stress [20] physiological or pathological conditions.
In both tumorigenesis and cardiovascular pathology, calcium (Ca2+) is considerate to be an essential constituent vital to the healthy physiology and disease pathology of both tumor cells and myocytes [21–24]. In regulation of cellular proliferation and apoptotic death, AKT/AMPK pathway control the cell cycle by targeting on critical point of G2/S transition functioning along with Ras and Cyclin D1. With involvement of autophagic proteins including Beclin, LC3-1 and LC3-II, up-regulations of AMPK, phospholated pAKT and pmTOR powerfully link Akt/mTOR associated autophagy to osteogenic differentiation of human mesenchymal stem cells [25].
Recently, it confirmed that the VC process accompanies on expression alternation of vascular smooth muscle cells (VSMCs) contractile phenotype-related factors such as α-SMA, calponin-1, SM22α and others along with the imbalance of a variety of calcification promoting factors including ALP, Runx2, BMPs, OCN, Collagen I and their inhibitors [26, 27]. Osteoblasts derived from VSMCs and mesenchymal stromal cells (MSCs) are regulated by autophagy [28, 29] and promotes transition of calcification signals for mineralization in the vessel wall of the vascular structure [30–32]. Autophagy within the physiological range functions protective effect but pathologic autophagy generates excessively or less activation. In this paper, we elucidate that the clarification on the mechanism of autophagy regulated VC would provide valuable information for developing diagnostic strategy and anti-VC drug design targeting on autophagy.
Autophagy affects vascular calcification by interfering with the osteogenic differentiation of VSMCs
As defined as an active, highly controllable mineral deposition process, the pathological changes of VC involve intima and middle layer of blood vessels, mainly VSMCs included in vessels-wall structure [33]. In the VC process, VSMCs transform from contractile phenotype to osteogenic/chondral phenotype directly or through synthetic inter-type [15]. Currently known related signaling pathways include ERα, β-catenin, HIF-1a/PDK4, p62, miR-30b, BECN1, mTOR, SOX9, GHSR/ERK, AMPK, Elastin [34–39]. The definite mechanism of osteoblastic differentiation of VSMCs is critical for vascular calcification.
Autophagy reduces calcification by inhibiting the osteogenic differentiation of VSMCs
Liang, et al. proved that long non‑coding RNA‑ANCR promoted the expression of LC3 and Atg5 in β‑GP‑induced VSMCs, and inhibited osteoblastic differentiation of VSMCs. The ANCR may attenuate arterial calcification through activating autophagy that inhibits osteogenic differentiation of VSMCs [40]. The autophagy inhibitor 3-MA or knockout of Atg5 increased calcium deposition, whereas the autophagy inducer valproic acid reduced VSMC calcification [31]. Yuan and colleagues approved that oestrogen inhibited the osteoblastic differentiation of VSMCs by promoting autophagy through the ERα but not ERβ signaling pathway [34].
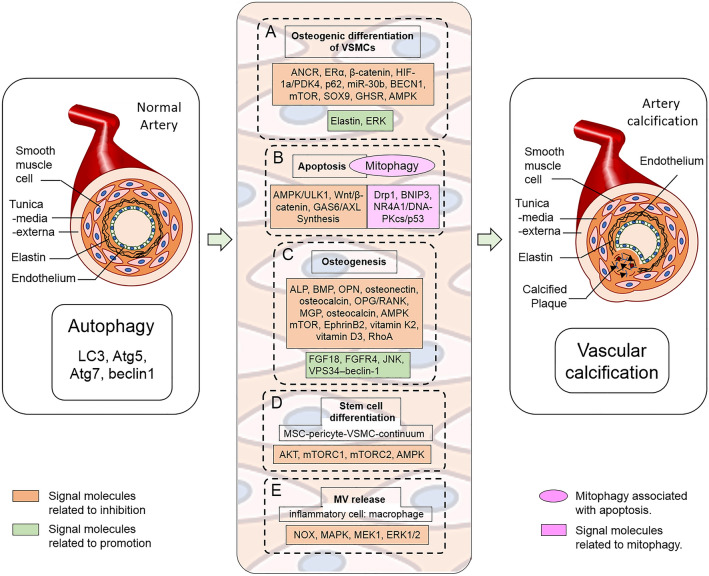
Statins display various protective effects against VSMC proliferation and inflammation in cardiovascular remodeling [41] and inhibit calcification of atherosclerotic plaques in the apoE-deficient mice [42]. Results from clinical trials suggest an association of statins usage with slow progression of calcific aortic stenosis, and coronary artery calcification [43, 44]. Liu and colleagues found inhibitory effect of atorvastatin on calcification is caused by inducing autophagy by using 3-MA, chloroquine, NH4Cl and bafilomycin A1. Their data approved that atorvastatin can protect VSMC differentiation from TGF-β1-stimulated calcification through suppression of β-catenin pathway [35]. Their data are consistent with the recently results that atorvastatin could reduce arterial calcification and plasma calcium concentration [45] (Fig. 1).
Fig. 1.
Signaling molecules linking autophagy to vascular calcification. A Autophagy affects VC by interfering with the osteogenic differentiation of VSMCs. B Autophagy may affect VC by inhibiting apoptosis. C VC is a tightly cell-regulated pathological process that resembles osteogenesis. D The effect of autophagy regulation in stem cells on VC. E MV involved in autophagy regulation of VC
Recently, it is approved that AGEs could increase alkaline phosphatase (ALP) and accelerate the calcification of VSMCs [46]. AGE-BSA treatment on VSMCs improved the expression of PDK4 via HIF-1α upregulation. The AGE-BSA incubation promoted expression increase of LC3-II and decrease of p62 protein levels. The treatment could enhance autophagic flux mediated by mRFP-GFP-LC3 adenovirus, make co-localization of LC3-II and LAMP-1, and eventually augmenter the number of autophagosome under TEM. HIF-1a/PDK4 pathway was activated in the process of AGEs-induced autophagy of VSMCs, which reduced the expression of the Runt-related transcription factor (RUNX2) and presented protective effects against VC induced by AGEs [36].
Some miRNAs are implicated in proliferation, development, and function of VSMCs, and directly involved in pathological calcification [47]. miR-30b regulates the Runx2 expression and plays an important role in VC as a common feature in patients with CKD [48]. More studies have highlighted the central role of miR-30b in high Pi level-induced autophagy via the regulation of BECN1, which suggested miR-30b as possible target for the treatment of vascular diseases [49]. Li, et al.clarified that restoring miR-30b can promote autophagy while inhibiting VC by modulating mTOR signaling pathway in β‐glycerophosphate induced VSMCs. miR‐30b negative regulates SOX9 while restoring miR‐30b in cell can increase the mitochondrial membrane potential (MMP) in β‐glycerophosphate‐induced VSMCs [37].
Hormonal ghrelin prevents osteoblastic transformation and mineralization of VSMCs mediated by GHSR/ERK signaling pathway [38]. Ghrelin application increases the expression of LC3 and beclin1 indicated autophagy, while 3-MA delays the ameliorative effect of ghrelin on VC. The protein levels of p-AMPK are promoted by the hormonal treatment, and AMPK inhibitor, compound C blocks the effect of ghrelin on VC and autophagy. In animal model, the hormone promotes autophagy in VC aorta and activates AMPK pathway meanwhile. Improved autophagy was detected following the activation of AMPK, which resulted in VC amelioration [50].
Autophagy can promote the osteogenic differentiation of VSMCs within diverse circumstances
The co-relationship between calcification and autophagy indicates that autophagy is one target for inhibition of VSMC calcification. Different from the inhibitory effect of autophagy on the osteogenic trans-differentiation of VSMCs, indoxyl sulfate stimulates the autophagy pathway through downregulating the expression of SET domain encompassing lysine methyltransferase 7/9. Subsequently, it can induce osteoblast differentiation and matrix mineralization of VSMCs [51] (Table 1).
Table 1.
Autophagy involved vessel calcification through inhibition and promotion cellular pathways
| Functions | Targeted genes | Animal models and cell culture | Involved genes |
|---|---|---|---|
| VSMC osteogenic differentiation | |||
| Autophagic inhibition | ANCR [34] |
Animal model High calcitriol-induced mice model /male C57BL/6 Cell culture Mouse VSMCs of passages 3–8 |
Atg5, BMP-2, Cbfa1/Runx2, LC3-I/II |
| ERα [28] |
Animal model 6-week-old female C57/BL6 mice were ovariectomized under anaesthesia (by Nembutal 40 mg/kg i.m.). Cell culture Mouse vascular smooth muscle cells (mVSMCs) were acquired from 8-week-old female C57/BL6 OVX mice or mice with intact ovaries. Patient tissue Renal arterial samples from a total of 10 pairs of uremic patients scheduled to undergo kidney transplantation and from healthy donors |
Atg5, ALP, Cbfa1/Runx2, ERα, LC3-I/II | |
| β-catenin [29] | Cell culture VSMCs from Male Sprague–Dawley (SD) rats aorta | Atg5, ALP, BMP-2, Beclin-1, Collagen I, Histone H2B, LC3, Osteocalcin, β-catenin | |
| HIF-1a, PDK4, p62/SQSTM1 [30] | Cell culture VSMCs from Male SD rats aorta | Cbfa1/Runx2, HIF-1a, LC3, PDK4, p62/SQSTM1 | |
| BECN1 [43] | Cell culture VSMCs from the descending thoracic aorta of rats, 293 T cells | Atg4b, Atg5, Atg7, Atg12, Atg16, BECN1, LC3B, LC3I/II, ULK1, VPS34 | |
| mTOR, SOX9 [31] |
Animal model 5‐weeks‐old chronic kidney disease rats (5/6 nephrectomy) Cell Culture VSMC lines |
Beclin-1, Cbfa1/Runx2, LC3, LC3-II, miR-30b, mTOR, p‐mTOR, SOX9, S6K1, p‐S6K1 | |
| GHSR/ERK [32] | Cell culture Mouse VSMCs | ALP, Cbfa1/Runx2, ERK, p-ERK, GHSR, JNK, p-JNK, p-p38, p38 | |
| AMPK [44] |
Animal model 3-month-old male SD rats Cell culture Primary VSMCs from thoracic aortas of male SD rats |
AKT, p-AKT, Beclin-1, LC3, mTOR, p-mTOR | |
| Autophagic promotion | ERK [47] |
Animal model 6-week-old ApoE−/− mice (C57BL/6 J genetic background) Cell culture Human VSMCs (HA-VSMC, CRL-1999, ATCC, Manassas, VA, USA) |
ALP, Beclin-1, DUSP5, ERK1/2, p-ERK1/2, LC3, LC3-I/II, mTOR, p-mTOR, p62/SQSTM1 |
| Autophagic/ mitophagic apoptosis | |||
| Autophagic inhibition | AMPK/mTOR/ULK1 [54] | Cell culture VSMCs from the aortas of 4-week-old SD rats | ALP, AMPK, p-AMPK, BMP-2, Cbfa1/Runx2, Caspase 3, Cleaved caspase 3, LC3-II, LC3-I, MGP, p62/SQSTM1, α-SMA, mTOR, ULK1, p-ULK1 |
| Wnt/β-catenin [55] | Cell culture Primary HASMCs (human aortic smooth muscle cells) | BMP-2, Beclin-1, Bax, Bak, Bcl-2, Cbfa1/Runx2, β-catenin, Caspase 9, Caspase 3, LC3-II, p-mTOR, OPN, OPG, p62/SQSTM1, α-SMA | |
| GAS6/AXL synthesis [56] | Cell culture Rat VSMCs | Axl, GAS6, LC3-II | |
| Autophagic promotion | Drp1, BNIP3, NR4A1/DNA-PKcs/p53 [59] |
Animal model 6-week-old male Wistar rats Cell culture Primary VSMCs from 6-week-old SD rat thoracic aortas |
Bax, Bcl-2, BNIP3, BMP-2, Caspase 3, Cbfa1/Runx2, Drp1, DNA-PKcs, LC3, Mff, NR4A1, OPA1, p62/SQSTM1, p53, α-SMA, TOMM20, TOMM40, VDAC1 |
| Osteogenesis | |||
| Inhibition on osteoblast/osteoclast | AMPK mTOR [73] | Cell culture Primary human chondrocytes | ALP, Adiponectin, AMPK, p-AMPK (Thr172), Beclin-1, Caspase 3, Caspase 9, LC3-I, LC3-II, mTOR, p- mTOR, PARP |
| EphrinB2, RhoA [74] |
Animal model Dmp1Cre mice (Tg(Dmp1Cre)1Jqfe), EphrinB2-floxed (Efnb2tm1And) mice Cell culture Ocy454 cells and Kusa 4b10 cells |
BNIP3, Eps8l1, Efnb2, Fam134b, Fbxo32, Klf1, Lama2, LC3-I, LC3-II, Pthlh, Peg3, RhoA, Trim63, Tspo2, Unc5a | |
| Promotion on osteoblast/osteoclast | FGFR4, JNK, VPS34, beclin-1 [85] |
Animal modelAtg7fl/fl, GFP–LC3 mouse, Prx1-Cre, Col2a1-Cre, Fgf18- and Fgfr3-knockout mice, Fgfr4-knockout mice Cell culture GFP–LC3 primary chondrocytes, Fgf18+/− chondrocytes |
Atg7, AKT, p-AKT, AMPKa, p-AMPKa, Beclin-1, p-BCL2, Collagen II, ERK1/2, p-ERK, FGFR3, FGFR4, GOLPH3, HA, Histone H3, JNK, p-JNK, p–c-JUN, LC3B, mTORC1, p-mTORC1, PDI, p38, p-p38, p62/SQSTM1, P70S6K, p-P70S6K, VPS34, 4EBP1, p-4EBP1 |
| Regerentation (MSC-pericyte-VSMC-continuum) | |||
| Autophagic inhibition | AKT, mTORC1, mTORC2 [89] |
Animal model Male 10-week-old C57BL/6JRccHsd mice Cell culture Human bone marrow aspirates MSC |
AKT, Bcl-2, Cleaved caspase 3, Calponin, Cbfa1/Runx2, Collagen IIA1, Collagen III, ERK1/2, ERK1/2Thr202/Tyr204, LC3B, MLCK, mTOR, Osteopontin, Osterix, p16INK4a, p62/SQSTM1, pp70S6KThr389, pp70S6KThr421/Ser424, pAKTSer473, pAKTThr308, p70-S6, Rictor, Raptor, SMA, SM22α |
| AMPK [90] |
Animal model 6-weeks-old male SD rats Cell culture Tenocytes isolated from 3-week-old SD rats achilles tendons |
Cleaved caspase 3, Cleaved caspase 9, LC3A/B, p62/SQSTM1, p53, p21 | |
| Cellular derived MV (Macrophage inflammatory) | |||
| Autophagic inhibition | NOX-1, MAPK, MEK1, ERK1/2 [96] | Cell culture Primary rat VSMC from the descending thoracic aorta of CKD-MBD, Cy/ + rat | AT1R, Annexin II, Annexin V, Annexin VI, BMP-2, Cbfa1/Runx2, CD9, CD63, CD81, p-ERK1/2, Fetuin-A, p-MEK1, Myocardin, NOX-1, NOX-4, Osteocalcin, SM22α, SOD-1, SOD-2 |
Low dietary potassium can induce elevation of intracellular calcium, activate intracellular calcium signaling-mediated CREB and autophagy, and further promote VSMC osteoblast differentiation and calcification [52]. The occurrence and development of VC symptom caused by autophagy were linked to autophagy-induced degradation of elastin [39].
Interestingly, in osteogenic differentiation of VSMCs, autophagy can play an opposite role within diverse circumstances. Besides the above, two independent teams approved that IV application of astragaloside effectively inhibit autophagy and mineralization in VSMCs, in which the inhibition is accomplished within the involvements of the ERK signaling pathway mediated by lncRNA H19 and DUSP5 [53] and mainly associated with mesenchymal stromal cells (MSCs) [25]. The biological circumstances on osteogenic differentiation left a critical challenge for drug design when developing anti-VC targeting autophagy pathway.
Autophagy affects vascular calcification by inhibiting apoptosis
It was proved that apoptotic cells can compose a nidus for the deposition of calcium-phosphate crystals [54]. The studies that apoptotic bodies form a nidus to nucleate apatite from dying VSMC [16] reflect the importance of VC promoted apoptosis, which indicated a possible mechanisms initiating the VSMC calcification process [55].
Autophagy inhibits apoptosis and vascular calcification
Many works linked apoptosis and VSMC calcification in both human sample and animal models. For instance, massive apoptotic cell death found in both human and animal atherosclerotic plaques [56], suggests that apoptosis could promote calcification of providing matrix through the release of apoptotic bodies in nidus along with nucleation sites of VC. In calcification model of uremia, apoptosis is positively detected with calcification of VSMCs, which always occurs before calcification. The observation exhibited that in which apoptotic bodies were normally found to encompass with high concentrations of calcium by accumulating on extracellular matrix (ECM), and eventually leading to calcification [57].
In hyperphosphate-induced VC, inhibition of calcium aggradation can be attained by inhibiting apoptosis and potentiating autophagy and many hormonal molecules exhibits their critical roles [58–61]. Melatonin protected VSMCs against apoptosis and attenuated β-GP-induced VSMC calcification via autophagy stimulation associated to an AMPK/mTOR/ULK1 signaling pathway [59]. Bavachin suppresses apoptosis and calcification effects in HASMCs. Mechanism of the above hormonal effect is dependent on Atg7/mTOR-mediated autophagy pathway and suppression of Wnt/β-catenin signaling [60]. Cozzolino group also claimed the related role of autophagy and apoptosis in the iron citrate preventing calcium deposition in high Pi-calcified VSMC associated with an iron-induced positive modulation of GAS6/AXL synthesis. They demonstrated that iron citrate arrests further high Pi-induced calcium deposition through an anti-apoptotic action and induction of autophagy on established calcified VSMC [61].
While a different in vitro model was established to delay high phosphate-induced VC progression, the group treated rat aortic VSMCs with high Pi in a repeated and short suspensions of high Pi treatment (intermittent suspension, IS). The treatment generates significant inhibition on high Pi calcification. Their data further approved that the inhibition on apoptosis is carried out through the preservation of AXL protein levels [62] and enhanced autophagy plays a protective role in arterial calcification through inhibiting apoptosis.
Mitophagy partially reverse mitochondrial disorder of vascular calcification
After mitochondrial toxicity is induced, the damaged mitochondria will be wrapped in a double-layer membrane structure to form autophagosomes, which are further degraded by lysosomes, otherwise the intrinsic apoptotic pathways will be activated. Its on-site accumulation promotes crystallization as nucleation of calcium phosphate crystals for further VC plaques [63]. Other data demonstrated that lactate impaired mitochondrial function inducing oxidative stress and apoptosis during VSMC calcification.
The Drp1‑related mitochondrial fission promoted by lactate through NR4A1 upregulation, while NR4A1 suppressed the autophagic flux and BNIP3‑mediated mitophagy, which were by p53 regulated phosphorylation. The regulation of Drp1 and BNIP3 is further related to the NR4A1/DNA‑PKcs/p53 pathway in the pathological plaques [64]. BNIP3-mediated mitophagy could partially reverse mitochondrial disorder, excessive oxidative stress and enhanced apoptosis, which plays a protective role against VSMC calcification in the presence of lactate [65]. This phenomenon suggests that, to some extent, autophagy/mitophagy can avert the activation of apoptotic pathways by the removal of damaged mitochondria [66].
As two critical catabolic processes that assist preserve cell and tissue homeostasis [67, 68], autophagy and apoptosis are highly related in deciding cell fate. Apoptosis stringently related to autophagy could be considered the result of the failure of autophagy to re-establish a physiological balance for the cells involving in survival. Autophagy can promote cell survival, but under certain conditions, autophagy also protected cells from necrosis via promoting apoptosis. The autophagy played a role in either promoting apoptosis or inhibiting apoptosis [68]. Therefore, the precarious issue is how to determine the range of moderate and appropriate mitophagy for VC.
VC associated osteogenic resembling and cellular pathological autophagy
The normal distribution where calcium is mineralized in the human body are bones and teeth meanwhile it becomes VC when calcium is excessively deposited on the blood vessel wall [28]. In the process of VC, the cells within vascular wall are transformed into an osteoblast-like phenotype, and begin to synthesize and secrete a variety of proteins related to bone formation, such as ALP, bone morphogenetic protein (BMP), osteopontin (OPN), osteonectin, osteocalcin, etc. [69]. Calcium nodules are formed in the extracellular matrix or cytoplasm of these cells, which are tightly associated with non-bone osteogenesis or autophagy related mineral resembling [70].
Vascular calcification and osteoporosis
While VC patients with high risk are often accompanied with osteoporosis, Matrix Gla protein (MGP) and osteocalcin are important factors for their regulation. The normal calcium balance in the human body guarantees the amount of calcium required in bones and teeth, but not abnormal calcium deposition in other locations such as blood vessels and internal organs [71]. When this calcium associated metabolism is disturbed, it will lead to a series of diseases, such as osteoporosis due to excessive loss of calcium in the bones, and dominant gain of calcium on the blood vessel wall.
VC often occurs simultaneously with low bone mineral density or poor bone turnover [72]. In general consideration, the balance and metabolism of calcium are closely related to vitamin K2, vitamin D3, MGP and osteocalcin [73]. The study of VC mechanism often involves changes in the expression of proteins related to bone development, such as OPG/RANK, OPN, MGP, BMP, etc. [69], in which the proteins plus blood calcium are cellular and mechanically resembled bone tissue architecture during osteogenesis. This bone-like structure formation is based on the structure and scaffold of blood vessels, and depending on osteoblasts differentiated from angiogenic pericytes or blood-borne mesenchymal cells surrounding the vasculature [74]. Up to date, many factors regulating bone mineralization are proved in calcified plaques [75, 76].
Autophagy exhibits variable effects in the metabolism of bone tissue and vessel
Property of autophagic recycling damaged organelles is highly related to bone metabolism during the dynamic synthesis and degradation process in bone [77]. Autophagy can display variable effects in the calcification of chondrocytes/osteocytes. Then, AdipoRon can activate autophagy of Osteoarthritis (OA) chondrocytes through the AMPK mTOR pathway, and as well improve autophagy contribution to suppress calcification in OA chondrocytes [78].
Using osteocyte differentiation approach, Vrahnas and colleagues collected data within in vitro system on murine stromal cell lines, Ocy454 cells and Kusa 4b10 cells compared to Dmp1Cre mice (Tg(Dmp1Cre)1Jqfe) and EphrinB2-floxed (Efnb2tm1And) mice. The data exhibited that significant mineral deposition in Dmp1Cre.Efnb2f/f bone along with massive increase of autophagosomes [79] and EphrinB2 deletion in osteocytes generated defected mice with brittle bones. Their result approved that Osteocytic EphrinB2 could limits autophagy through RhoA, which could be responsible for limiting mineral accumulation and carbonate substitution within the bioapatite matrix and restraining collagen fiber compaction [79]. Subsequently, further investigations unveiled that EphrinB2 deficiency in bones dysregulated many genes including Fam134b [80], Fbxo32 [81], Lama2 [82], Bnip3 [83], Peg3 [84], Eps8l1 [85], Klf1 [86], Tspo2 [87], and Unc5a [88], which is specifically related to a series of autophagy processes, including mitophagy and ER-phagy.
Recently, a study claimed that autophagy plays a critical role in promoting vascular calcification within osteoblast differentiation and mineralization using vitamin K2-induced MC3T3 E1 cells [89]. During post-natal bone development, autophagy is induced in growth-plate chondrocytes during post-natal bone development via regulating the secretion of type II collagen (Col2). When FGFR4 and JNK-dependent activation, the autophagy initiated complex VPS34 and beclin-1 [90]. Therefore, on compression on how autophagy regulating VC, further investigations can plagiarize the above information from bone formation [80–88].
The effect of autophagy regulation in stem cells on vascular calcification
Because both VSMCs and osteoblasts are mesenchymal originated, many works focus on the mechanism of VC on the correlation with stem cells. Interestingly, as MSCs have a dual role as progenitors to osteoblasts and pericytes further to develop to VSMC, some studies found that cells with MSC phenotype in the adventitia of arteries are the major source of osteoblast-like cells in intimal and medial calcification [29]. The uremic milieu causes osteoblastic differentiation of MSC and calcification [91], indicating loss of vascular progenitor properties.
Recently, Carracedo and his colleagues discovered that initiated autophagy in calcific aortic valve stenosis (CAVS) confers protection of valvular interstitial cells (VICs). Their data suggest that the upregulation of autophagy observed in the calcified tissue of these valves serves as a compensatory and pro-survival mechanism to protect valves from calcification [92]. Their results are also supported by another result that VIC autophagy could prevent calcification via pro-osteogenic signaling [93]. Hegner and colleagues discovered that mTORC1 and mTORC2 pathways show different regulatory roles in cell fate during the osteoblastic differentiation from MSCs. Furthermore, other studies demonstrated that blockade of autophagy can exacerbate calcification of differentiated MSC. Inhibition of AKT signaling or genetic depletion of mTORC2 abrogate the protective effect of rapamycin on MSC calcification. And enhanced mTORC2 signaling is sufficient in this protection effect from MSC against calcification [94]. Autophagy could be attributed a key role in the transition from undifferentiated MSC to osteoblast-like calcifying cells.
In a recent report, a study shows that, in tendon-derived stem cells (TDSCs), pioglitazone increased the ratio of LC3B/LC3A and decreased that of P62 expression, and performed as an agonist to promote autophagy via modulation of the AMPK/mTOR pathway. Pioglitazone treatment can induce autophagy flux in AGEs-treated TDSCs, which possesses anti-apoptosis, anti-senescence, and anti-ossification effects [95]. Based on the discussion above, targeting on differentiated progenitor cells such as VSMC and osteoblast-like cells could maintain and resort the endogenously physiological MSC function. Enabling protective cell fate patterns in the MSC-pericyte-VSMC-continuum could be an innovative approach for treatment of VC.
MV involved in autophagy regulation of vascular calcification
As an effective approach based on recent investigation, MV can be transmitted among co-cultured cells through endocytosis and induced cell-cell communication. When extracellular MV exosomes containing low fetuin-A content were added to recipient VSMC, the calcification was increased upon these cells. The increase in calcium induced by cellular derived MV is partly attributable to the activation of NOX and MAPK (MEK1 and Erk1/2) signaling pathway [96].
MVs formed from VSMCs and macrophages under atherosclerotic conditions mainly, are calcified and released into the collagen-rich matrix within the intima of vessel [19, 97]. Macrophages indirectly promote mineral formation by producing the inflammatory cytokines while lipid oxidation products promote vascular cell mineralization. Xu, et al emphasized that autophagy would be an endogenous protective mechanism counteracting VC under hyperphosphatemia. The autophagy inhibition leads to increased MV release rather than cell apoptosis, and the inhibition promoted Pi-induced MV release and increased ALP activity may be the cause of calcification [31]. Other study on the calcification of aortic leaflets claimed that autophagy resulted in the release of MVs in early degenerative aortic valves, which attract inflammatory cells and triggered calcification of the valve [98, 99].
Depending on various induction signals, the origin and selected content in the MVs of autophagy promoted VSMC exocytosis are different [31, 32], and MVs are considered the first nidus for mineralization in the vessel wall [30]. Therefore, as an effective hydroxyapatite mediator, the mechanism of MV autophagy release in the initiation and development of VC needs more detailed research.
Current clinical trial involved with autophagy related treatment for VC
Autophagy related treatment have been used for halting many cancer development involved with autophagic pathways including in both animal models and human samples [100–103]. Strong potential drugs against diverse tumors were designed and applied as anti-cancer therapy. Autophagy-targeting drugs such as autophagy inhibitors Choloroquine and Bafilomycin A1 targeting on endosomal acidification, and 3-Methyladenine and LY294002 targeting PI3K pathway currently approved for use in the treatment of solid and non-solid malignancies [100]. However, up to date, only one drug SNF472 against autophagy recently were used on Phase 2 treatment for treatment against cardiovascular calcification in patients with actual strong positive results [103, 104].
As a derivative of phytic acid, SNF472 (hexasodium salt of phytate) play a critical role as a potential treatment for Alzheimer's disease targeting on autophagy- associated proteins (beclin-1 and LC3B) [105]. Currently, using SNF472 as a calcification inhibitor, a clinical Phase II CaLIPSO trial (EudraCT 2016–002834–59) for the treatment of cardiovascular calcification is completed. Based on double-blind and placebo-controlled Phase II trial, computed tomography scan unveiled that, in randomized three groups including SNF472 300 mg (n=92)/SNF472 600 mg (n=91)/or placebo control (n=91), two SNF472 dosages significantly slowed down of both the accumulation of coronary artery calcium and the development of aortic valve calcification [103, 104]. This favorable data highlights strong possibility to develop more practical and high efficient approach with other drugs targeting on the pathway of the molecular mechanism of autophagy in VC associated cardiovascular disorders.
With the above promising results, it would be rational to accomplish more clinical trials associated with autophagic proteins (Table 2) based on massive positive data obtained animal models. Providentially, along with endosomal acidification targeted and PI3K pathway targeted autophagy inhibitors, MAPK pathway associated inhibitors including SB202190 and SB203580 also be available for diverse VC related trials. The advantage should be employed in which many autophagy inhibitors are FDA approved drugs that can be used in other diseases [100–102].
Table 2.
Autophagy related proteins in human and their functions in vascular calcification
| # | Autophagy proteins involved | Functions in VC process | PubMed ID | Chromosome location/band | Transcript (bp) | CDS (bp) | Peptides (AA) | References |
|---|---|---|---|---|---|---|---|---|
| 1 | LC3-I transcript variant 1 | Cysteine protease cleaved LC3 I from LC3 inhibits osteogenic differentiation of VSMCs | NM_032514 | Chromosome 20: 34,546,854–34,560,345 forward strand; 20q11.22 | 964 | 366 | 121 | Peng et al. [28]; |
| LC3-I transcript variant 2 | NM_181509 | 971 | 378 | 125 |
Zhang et al. [34]; Song et al. [47]; Xu et al. [90] |
|||
| 2 | Atg5(Autophagy related 5) | Knocking down Atg5 expression significantly upregulates β-GP-induced Runx2 expression and ALP activity along inhibition of osteogenic differentiation of VSMCs | JQ924061.1 | Chromosome 6: 106,045,423–106,325,791 reverse strand; 6q21 | 828 | 828 | 275 |
Dai et al. [25]; Zhang et al. [34] |
| 3 | LC3-II | Along with enhancing autophagic flux, LC3-II augmenter the number of autophagosome by reducing the RUNX2 expression | NM_022818 | Chromosome 16: 87,383,953–87,404,779 forward strand; 16q24.2 | 2147 | 378 | 125 |
Peng et al. [28]; Yang et al. [30]; Song et al. [47]; Xu et al. [90] |
| 4 | p62 (SQSTM1) | By reducing the RUNX2 expression, p62 augmenter the number of autophagosome | M88108 | Chromosome 5: 179,806,398–179,838,078 forward strand; 5q35.3 | 2685 | 1332 | 443 | Yang et al. [30] |
| 5 | Beclin1 transcript variant 1 | As an up-regulator of autophagic pathway, beclin1 decreases expression of Runx2 and Msx2 | NM_003766 | Chromosome 17: 42,810,134–42,833,350 reverse strand; 17q21.31 | 2131 | 1353 | 450 |
Xu et al. [31]; Wang et al. [43] |
| NM_001313998 | 2109 | 1353 | 450 | |||||
| NM_001313999 | 1840 | 1068 | 355 | |||||
| 6 | Atg7 transcript variant 4 | As an upstream autophagy-related gene of LC3, Atg7 promotes the conversion of LC3-I to LC3-II and prevention of calcification | NM_001349232 | Chromosome 3: 11,272,309–11,557,665 forward strand; 3p25.3 | 5333 | 2112 | 703 | He et al. [58] |
| Atg7 transcript variant 5 | NM_001349233 | 5087 | 2112 | 703 | ||||
| Atg7 transcript variant 7 | NM_001349235 | 5224 | 2112 | 703 | ||||
| 7 | VPS34 transcript variant 1 | Forming VPS34–beclin-1 complex for autophagy initiation and promoting vascular calcification | NM_002647 | Chromosome 18: 41,955,234–42,087,830 forward strand; 18q12.3 | 9415 | 2664 | 887 | Cinque et al. [85] |
| VPS34 transcript variant 2 | NM_001308020 | 9226 | 2475 | 824 |
Conclusion and perspective
The maintenance of the normal structure of blood vessels and the regulation of its functions are critical for circulation system which is tightly related to autophagy. Within a certain criteria, autophagy activation as a protective affection on VSMCs can promote cell survival, lead to enhanced cell proliferation, migration, and extracellular matrix secretion, and reduce calcification. Removing cell debris such as misfolded proteins and dysfunctional organelles that can cause senescence and apoptosis, autophagy plays an important role in maintaining the adaptability of juvenile cells [95]. Reversely, when autophagy is inhibited, lack of autophagy leads to accumulation of harmful substances in cells, cell aging, changes in vascular structure, vasomotor function becomes abnormal, and the increasing incidence of VC. When autophagy is beyond the scope of its beneficial effects and/or over-activated, cell and organelle damage within VSMCs could lead to the vascular calcification accompanying with triggering cell death, and further accelerating the occurrence of VC. Autophagy plays an intricate and often distinct role under various pathological conditions.
The effects of autophagy on VC appears to be complicated, which depend on degree of autophagy associated with disease status, location, and the surrounding microenvironment. Autophagy activation in the presence of acute pathological damage is generally considered to be protective, resulting in degradation of dysfunctional cellular components and maintenance of cell homeostasis. Reversely, some chronic diseases induce sustained autophagy which may be detrimental, since defective autophagy can activate the apoptotic pathway, damage important organelles and cause cell apoptosis. In order to develop effective and unique approaches to slow down or eliminate VC targeting on VC-related autophagy, the functional and regulatory genes in osteogenesis including the bone formation such as Fam134b, Klf1 and Bnip3, and the MSCs differentiation such as ALP, Runx2 and BMPs could be efficient candidates. The more intensive investigation on precise chick-point between these beneficial function and apoptotic death occurred on vessel wall, would be critical in further study in this field.
Acknowledgements
Not applicable.
Abbreviations
- AKT/PKB
Protein kinase B
- AMPK
AMP-activated protein kinase
- Atg
Autophagy-related gene
- ANCR
Anti-differentiation noncoding RNA
- AGEs
Advanced glycation end products
- ALP
Alkaline phosphatase
- apoE
Apolipoprotein E
- α-SMA
Alpha smooth muscle actin
- BMP
Bone morphogenetic protein
- BSA
Bovine serum albumin
- BNIP3
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- β-GP
β-Glycerophosphate
- CREB
CAMP response element-binding protein
- Col2
Type II collagen
- CAVS
Calcific aortic valve stenosis
- CKD
Chronic kidney disease
- DUSP5
Dual specificity protein phosphatase 5
- Drp1
Dynamin-related protein 1
- DNA‑PKcs
DNA-dependent protein kinase, catalytic subunit
- ERα
Oestrogen receptor alpha
- ERβ
Oestrogen receptor beta
- ER-phagy
Microautophagic degradation of the ER
- ECM
Extracellular matrix
- ERK1/2
Extracellular signal-regulated kinases1/2
- FGF23
Fibroblast Growth Factor 23
- FGF18
Fibroblast growth factor 18
- FGFR4
Fibroblast growth factor receptor 4
- GFP
Green fluorescent protein
- GAS6
Growth arrest-specific 6
- GHSR (ghrelin receptor)
Growth hormone secretagogue receptor
- HIF-1α
Hypoxia-inducible factor 1-alpha
- HASMCs
Human Aortic Smooth Muscle Cells
- IS
Intermittent suspension
- JNK-dependent
C-Jun N-terminal kinases
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- LAMP-1
Lysosomal-associated membrane protein 1
- lncRNA H19
Long non-coding RNAs
- MAPK
Mitogen-activated protein kinase
- mTOR
Mammalian target of rapamycin
- mRFP
Monomeric red fluorescent proteins
- Mitophagy
Degradation of mitochondria
- MScs
Mesenchymal stromal cells
- mTORC1
Mammalian target of rapamycin complex 1
- mTORC2
Mammalian target of rapamycin complex 2
- MV
Matrix Vesicle
- MMP
Mitochondrial membrane potential
- MGP
Matrix Gla protein
- NR4A1
Nuclear receptor subfamily 4 group A member 1
- NOX
NADPH oxidase
- OPN
Osteopontin
- OCN
Osteocalcin
- OPG
Osteoprotegerin
- OA
Osteoarthriti
- PI3K
Phosphoinositide 3-kinases
- p53
Tumor protein p53
- PDK4
Pyruvate dehydrogenase kinase 4
- PPi
Pyrophosphoric acid
- p62/SQSTM1
Sequestosome 1
- ULK
Unc-51-like kinase 1
- p53
Tumor protein p53
- RANK
Receptor activator of nuclear factor κB
- RhoA
Ras homolog family member A
- Runx2
Runt-related transcription factor 2
- SM22α
Smooth muscle 22 alpha
- TGF-β1
Transforming growth factor beta 1
- TEM
Transmission electron microscopy
- TDSCs
Tendon-derived stem cells
- VC
Vascular calcification
- VSMCs
Vascular smooth muscle cells
- VICs
Valvular interstitial cells
- 3-MA
3-Methyladenine
Authors’ contributions
XZ and HLC conceived, XZ, SNX and STY wrote the manuscript, XJL, LX, HJL, CXH, WQ, DZ, and XYS collected and analyzed references. XHX provided the recent information and analysis on the clinical trial in the manuscript. EM, HLC and XHX edited and finalized the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Key Program of Shaanxi Provincial Education Department (No. 20JS138 to XZ); the National Natural Science Foundation of China (No. U1932130 to H-LC, No. 31700699 to WQ); the Natural Science Basic Research Program Youth Project of Shaanxi Provincial Science and Technology Department (No. 2020JQ-885 to XZ); the Program of Shaanxi Administration of Traditional Chinese Medicine (No. 2019-ZZ-ZY009 to H-LC); the Key Program of Weiyang District Bureau of Science, Technology and Industry Information Technology (No. 201928 to H-LC); College Student Innovation Training Program of Xi’an Medical University (No. 121520012/S202011840012/202011840012 to S-TY), Doctorial Program from Xi’an Medical University (No. 2020DOC28 to XZ); X-HX is partially supported through NSFC Grant No. #31771377/31571273/31371256 and Ministry of Education Central Universities Research Fund GK201301001/201701005.
Availability of data and materials
Data and materials are available upon request to corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Zhou, Sui-Ning Xu and Shu-Tong Yuan contributed equally to this work
Contributor Information
Edward Moharomd, Email: edwh_mohard@jhmi.edu.
Xuehong Xu, Email: xhx0708@snnu.edu.cn.
Hui-Ling Cao, Email: caohuiling.jzs@xiyi.edu.cn.
References
- 1.Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37(2):191–204. doi: 10.1161/atvbaha.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 2017;91(4):808–817. doi: 10.1016/j.kint.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Lee IK, Jeon JH. Vascular calcification—new insights into its mechanism. Int J Mol Sci. 2020;21(8):2685. doi: 10.3390/ijms21082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesauro M, Mauriello A, Rovella V, Annicchiarico-Petruzzelli M, Cardillo C, Melino G, Di Daniele N. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281(5):471–482. doi: 10.1111/joim.12605. [DOI] [PubMed] [Google Scholar]
- 5.Ohtake T, Kobayashi S. Impact of vascular calcification on cardiovascular mortality in hemodialysis patients: clinical significance, mechanisms and possible strategies for treatment. Ren Replace Ther. 2017;3(1):1–11. doi: 10.1186/s41100-017-0094-y. [DOI] [Google Scholar]
- 6.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34(4):715–723. doi: 10.1161/atvbaha.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–3318. doi: 10.1002/cncr.31335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes WE, Beyer AM, Gutterman DD. Vascular autophagy in health and disease. Basic Res Cardiol. 2020;115(4):41. doi: 10.1007/s00395-020-0802-6. [DOI] [PubMed] [Google Scholar]
- 9.Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: A comprehensive review. Biomed Pharmacother. 2018;104:485–495. doi: 10.1016/j.biopha.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Deng Z, Ma Y, Jin J, Qi F, Li S, Liu C, Lyu FJ, Zheng Q. The role of autophagy and mitophagy in bone metabolic disorders. Int J Biol Sci. 2020;16(14):2675–2691. doi: 10.7150/ijbs.46627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colella B, Faienza F, Carinci M, D'Alessandro G, Catalano M, Santoro A, Cecconi F, Limatola C, Di Bartolomeo S. Autophagy induction impairs Wnt/β-catenin signalling through β-catenin relocalisation in glioblastoma cells. Cell Signal. 2019;53:357–364. doi: 10.1016/j.cellsig.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. doi: 10.1161/circresaha.117.311082. [DOI] [PubMed] [Google Scholar]
- 14.Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular aging. Circ Res. 2018;123(7):803–824. doi: 10.1161/circresaha.118.312208. [DOI] [PubMed] [Google Scholar]
- 15.Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114(4):590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87(11):1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 17.Viegas C, Araújo N, Marreiros C, Simes D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): challenging old concepts with new facts. Aging (Albany NY) 2019;11(12):4274–99. doi: 10.18632/aging.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone. 2017;100:87–93. doi: 10.1016/j.bone.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116(8):1312–1323. doi: 10.1161/circresaha.116.305012. [DOI] [PubMed] [Google Scholar]
- 20.Wei R, Enaka M, Muragaki Y. Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Sci Rep. 2019;9(1):10366. doi: 10.1038/s41598-019-46824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu MM, Seas A, Kiyani M, Ji KSY, Bell HN. A temporal examination of calcium signaling in cancer- from tumorigenesis, to immune evasion, and metastasis. Cell Biosci. 2018;8:25. doi: 10.1186/s13578-018-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Xu MM, Li Z, Shi M, Zhou X, Jiang X, Bryant J, Balk S, Ma J, Isaacs W, Xu H. Calcium and CaSR/IP3R in prostate cancer development. Cell Biosci. 2018;8:16. doi: 10.1186/s13578-018-0217-3. [DOI] [Google Scholar]
- 23.Zhou X, Li Z, Wang Z, Chen E, Wang J, Chen F, Jones O, Tan T, Chen S, Xu X, et al. Syncytium calcium signaling and macrophage function in the heart. Cell Biosci. 2018;8:24. doi: 10.1186/s13578-018-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xuehong Xu, Balk SP, Isaacs WB, Ma J. Calcium signaling: an underlying link between cardiac disease and carcinogenesis. Cell Biosci. 2018;8:39. doi: 10.1186/s13578-018-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52(1):524–531. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35(23):1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical. Circ Res. 2006;99(10):1044–59. doi: 10.1161/01.res.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 28.Caffarelli C, Montagnani A, Nuti R, Gonnelli S. Bisphosphonates, atherosclerosis and vascular calcification: update and systematic review of clinical studies. Clin Interv Aging. 2017;12:1819–1828. doi: 10.2147/cia.s138002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19(5):628–642. doi: 10.1016/j.stem.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanahan CM. Autophagy and matrix vesicles: new partners in vascular calcification. Kidney Int. 2013;83(6):984–986. doi: 10.1038/ki.2013.75. [DOI] [PubMed] [Google Scholar]
- 31.Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, Kong W, Zhu WG, Xu MJ, Wang X. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013;83(6):1042–1051. doi: 10.1038/ki.2012.482. [DOI] [PubMed] [Google Scholar]
- 32.Lee K, Kim H, Jeong D. Microtubule stabilization attenuates vascular calcification through the inhibition of osteogenic signaling and matrix vesicle release. Biochem Biophys Res Commun. 2014;451(3):436–441. doi: 10.1016/j.bbrc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Jaminon A, Reesink K, Kroon A, Schurgers L. The Role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. 2019;20(22):5694. doi: 10.3390/ijms20225694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng YQ, Xiong D, Lin X, Cui RR, Xu F, Zhong JY, Zhu T, Wu F, Mao MZ, Liao XB, et al. Oestrogen inhibits arterial calcification by promoting autophagy. Sci Rep. 2017;7(1):3549. doi: 10.1038/s41598-017-03801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D, Cui W, Liu B, Hu H, Liu J, Xie R, Yang X, Gu G, Zhang J, Zheng H. Atorvastatin protects vascular smooth muscle cells from TGF-β1-stimulated calcification by inducing autophagy via suppression of the β-catenin pathway. Cell Physiol Biochem. 2014;33(1):129–141. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- 36.Yang R, Zhu Y, Wang Y, Ma W, Han X, Wang X, Liu N. HIF-1α/PDK4/autophagy pathway protects against advanced glycation end-products induced vascular smooth muscle cell calcification. Biochem Biophys Res Commun. 2019;517(3):470–476. doi: 10.1016/j.bbrc.2019.07.102. [DOI] [PubMed] [Google Scholar]
- 37.Xu TH, Qiu XB, Sheng ZT, Han YR, Wang J, Tian BY, Yao L. Restoration of microRNA-30b expression alleviates vascular calcification through the mTOR signaling pathway and autophagy. J Cell Physiol. 2019;234(8):14306–18. doi: 10.1002/jcp.28130. [DOI] [PubMed] [Google Scholar]
- 38.Liang QH, Jiang Y, Zhu X, Cui RR, Liu GY, Liu Y, Wu SS, Liao XB, Xie H, Zhou HD, et al. Ghrelin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the ERK pathway. PLoS ONE. 2012;7(4):e33126. doi: 10.1371/journal.pone.0033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen K, Zhou X, Sun Z. Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension. 2015;66(5):1006–1013. doi: 10.1161/hypertensionaha.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Chen J, Meng Q, Li D, Hu FZ, Zhu YQ, Huang YY, Liu YN, Sun L, Liang QH. The protective effects of long non-coding RNA-ANCR on arterial calcification. J Bone Miner Metab. 2020;38(4):421–431. doi: 10.1007/s00774-019-01076-y. [DOI] [PubMed] [Google Scholar]
- 41.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18(11):1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibasaki M, Wang JG, Figueiredo JL, New SE, Quillard T, Goettsch C, Koga J, Sonoki H, Matsumoto J, Aikawa M, et al. Pitavastatin reduces inflammation in atherosclerotic plaques in apolipoprotein E-deficient mice with late stage renal disease. PLoS ONE. 2015;10(9):e0138047. doi: 10.1371/journal.pone.0138047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinauskienė R, Jonkaitienė R. Degenerative aortic stenosis, dyslipidemia and possibilities of medical treatment. Medicina (Kaunas) 2018;54(2):24. doi: 10.3390/medicina54020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuyama H, Langsjoen PH, Hamazaki T, Ogushi Y, Hama R, Kobayashi T, Uchino H. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Rev Clin Pharmacol. 2015;8(2):189–99. doi: 10.1586/17512433.2015.1011125. [DOI] [PubMed] [Google Scholar]
- 45.Tintut Y, Hsu JJ, Demer LL. Lipoproteins in cardiovascular calcification: potential targets and challenges. Front Cardiovasc Med. 2018;5:172. doi: 10.3389/fcvm.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Ma WQ, Han XQ, Wang Y, Wang X, Liu NF. Advanced glycation end products accelerate calcification in VSMCs through HIF-1α/PDK4 activation and suppress glucose metabolism. Sci Rep. 2018;8(1):13730. doi: 10.1038/s41598-018-31877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackenzie NC, Staines KA, Zhu D, Genever P, Macrae VE. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem Funct. 2014;32(2):209–216. doi: 10.1002/cbf.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louvet L, Metzinger L, Büchel J, Steppan S, Massy ZA. Magnesium attenuates phosphate-induced deregulation of a MicroRNA signature and prevents modulation of Smad1 and Osterix during the course of Vascular calcification. Biomed Res Int. 2016;2016:7419524. doi: 10.1155/2016/7419524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Sun YT, Xu TH, Sun W, Tian BY, Sheng ZT, Sun L, Liu LL, Ma JF, Wang LN, et al. MicroRNA-30b regulates high phosphorus level-induced autophagy in vascular smooth muscle cells by targeting BECN1. Cell Physiol Biochem. 2017;42(2):530–536. doi: 10.1159/000477602. [DOI] [PubMed] [Google Scholar]
- 50.Xu M, Liu L, Song C, Chen W, Gui S. Ghrelin improves vascular autophagy in rats with vascular calcification. Life Sci. 2017;179:23–29. doi: 10.1016/j.lfs.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Gu Y, Zhang H, Ning Y, Song N, Hu J, Cai J, Shi Y, Ding X, Zhang X. Amelioration of uremic toxin indoxyl sulfate-induced osteoblastic calcification by SET domain containing lysine methyltransferase 7/9 protein. Nephron. 2019;141(4):287–294. doi: 10.1159/000495885. [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Byon CH, Yang Y, Bradley WE, Dell’Italia LJ, Sanders PW, Agarwal A, Wu H, Chen Y. Dietary potassium regulates vascular calcification and arterial stiffness. JCI Insight. 2017;2(19):e94920. doi: 10.1172/jci.insight.94920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Z, Wei D, Chen Y, Chen L, Bian Y, Shen Y, Chen J, Pan Y. Association of astragaloside IV-inhibited autophagy and mineralization in vascular smooth muscle cells with lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol Appl Pharmacol. 2019;364:45–54. doi: 10.1016/j.taap.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Voelkl J, Lang F, Eckardt KU, Amann K, Kuro-O M, Pasch A, Pieske B, Alesutan I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. 2019;76(11):2077–2091. doi: 10.1007/s00018-019-03054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–634. doi: 10.1093/cvr/cvy007. [DOI] [PubMed] [Google Scholar]
- 56.Di M, Wang L, Li M, Zhang Y, Liu X, Zeng R, Wang H, Chen Y, Chen W, Zhang Y, et al. Dickkopf1 destabilizes atherosclerotic plaques and promotes plaque formation by inducing apoptosis of endothelial cells through activation of ER stress. Cell Death Dis. 2017;8(7):e2917. doi: 10.1038/cddis.2017.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez KJ, Piechura LM, Porras AM, Masters KS. Manipulation of valve composition to elucidate the role of collagen in aortic valve calcification. BMC Cardiovasc Disord. 2014;14:29. doi: 10.1186/1471-2261-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciceri P, Elli F, Braidotti P, Falleni M, Tosi D, Bulfamante G, Block GA, Cozzolino M. Iron citrate reduces high phosphate-induced vascular calcification by inhibiting apoptosis. Atherosclerosis. 2016;254:93–101. doi: 10.1016/j.atherosclerosis.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 59.Chen WR, Yang JQ, Liu F, Shen XQ, Zhou YJ. Melatonin attenuates vascular calcification by activating autophagy via an AMPK/mTOR/ULK1 signaling pathway. Exp Cell Res. 2020;389(1):111883. doi: 10.1016/j.yexcr.2020.111883. [DOI] [PubMed] [Google Scholar]
- 60.He HQ, Law BYK, Zhang N, Qiu CL, Qu YQ, Wu AG, Han Y, Song Q, Zheng WL, Liu Y, et al. Bavachin protects human aortic smooth muscle cells against β-Glycerophosphate-mediated vascular calcification and apoptosis via activation of mTOR-dependent autophagy and suppression of β-Catenin signaling. Front Pharmacol. 2019;10:1427. doi: 10.3389/fphar.2019.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciceri P, Falleni M, Tosi D, Martinelli C, Cannizzo S, Marchetti G, D'Arminio Monforte A, Bulfamante G, Block GA, Messa P, et al. Therapeutic effect of iron citrate in blocking calcium deposition in high Pi-calcified VSMC: role of autophagy and apoptosis. Int J Mol Sci. 2019;20(23):5925. doi: 10.3390/ijms20235925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciceri P, Elli F, Cappelletti L, Tosi D, Braidotti P, Bulfamante G, Cozzolino M. A new in vitro model to delay high phosphate-induced vascular calcification progression. Mol Cell Biochem. 2015;410(1–2):197–206. doi: 10.1007/s11010-015-2552-6. [DOI] [PubMed] [Google Scholar]
- 63.Ma WQ, Sun XJ, Wang Y, Zhu Y, Han XQ, Liu NF. Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis. Mol Cell Endocrinol. 2019;479:39–53. doi: 10.1016/j.mce.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y, Han XQ, Sun XJ, Yang R, Ma WQ, Liu NF. Lactate accelerates vascular calcification through NR4A1-regulated mitochondrial fission and BNIP3-related mitophagy. Apoptosis. 2020;25(5–6):321–340. doi: 10.1007/s10495-020-01592-7. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y, Ji JJ, Yang R, Han XQ, Sun XJ, Ma WQ, Liu NF. Lactate accelerates calcification in VSMCs through suppression of BNIP3-mediated mitophagy. Cell Signal. 2019;58:53–64. doi: 10.1016/j.cellsig.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 2015;116(8):1477–90. doi: 10.1161/circresaha.116.303790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gudipaty SA, Conner CM, Rosenblatt J, Montell DJ. Unconventional ways to live and die: cell death and survival in development, homeostasis, and disease. Annu Rev Cell Dev Biol. 2018;34:311–332. doi: 10.1146/annurev-cellbio-100616-060748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Li X, Yang Z, Zhu M, Xie J. Autophagy induced by low concentrations of crotonaldehyde promotes apoptosis and inhibits necrosis in human bronchial epithelial cells. Ecotoxicol Environ Saf. 2019;167:169–177. doi: 10.1016/j.ecoenv.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Alves RD, Eijken M, van de Peppel J, van Leeuwen JP. Calcifying vascular smooth muscle cells and osteoblasts: independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genomics. 2014;15(1):965. doi: 10.1186/1471-2164-15-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu YS, Gu Y, Jiang C, Chen L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J Cell Physiol. 2020;235(3):2220–2231. doi: 10.1002/jcp.29131. [DOI] [PubMed] [Google Scholar]
- 71.Wasilewski GB, Vervloet MG, Schurgers LJ. The bone-vasculature axis: calcium supplementation and the role of Vitamin K. Front Cardiovasc Med. 2019;6:6. doi: 10.3389/fcvm.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vassalle C, Mazzone A. Bone loss and vascular calcification: A bi-directional interplay? Vascul Pharmacol. 2016;86:77–86. doi: 10.1016/j.vph.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 73.van Ballegooijen AJ, Pilz S, Tomaschitz A, Grübler MR, Verheyen N. The Synergistic interplay between Vitamins D and K for bone and cardiovascular health: a narrative review. Int J Endocrinol. 2017;2017:7454376. doi: 10.1155/2017/7454376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sivaraj KK, Adams RH. Blood vessel formation and function in bone. Development. 2016;143(15):2706–2715. doi: 10.1242/dev.136861. [DOI] [PubMed] [Google Scholar]
- 75.De Maré A, Maudsley S, Azmi A, Hendrickx JO, Opdebeeck B, Neven E, D'Haese PC, Verhulst A. Sclerostin as regulatory molecule in vascular media calcification and the bone-vascular axis. Toxins (Basel) 2019;11(7):428. doi: 10.3390/toxins11070428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borland SJ, Morris TG, Borland SC, Morgan MR, Francis SE, Merry CLR, Canfield AE. Regulation of vascular smooth muscle cell calcification by syndecan-4/FGF-2/PKCα signalling and cross-talk with TGFβ. Cardiovasc Res. 2017;113(13):1639–1652. doi: 10.1093/cvr/cvx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S, Cailleteau L, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10(11):1965–77. doi: 10.4161/auto.36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan ZX, Tu C, Liu Q, Li SQ, Li YH, Xie P, Li ZH. Adiponectin receptor agonist AdipoRon attenuates calcification of osteoarthritis chondrocytes by promoting autophagy. J Cell Biochem. 2020;121(5–6):3333–3344. doi: 10.1002/jcb.29605. [DOI] [PubMed] [Google Scholar]
- 79.Vrahnas C, Blank M, Dite TA, Tatarczuch L, Ansari N, Crimeen-Irwin B, Nguyen H, Forwood MR, Hu Y, Ikegame M, et al. Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone. Nat Commun. 2019;10(1):3436. doi: 10.1038/s41467-019-11373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522(7556):354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 81.Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N, Carpi A, Carullo P, Pesce P, Sacerdoti D, et al. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest. 2014;124(6):2410–2424. doi: 10.1172/jci66339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gawlik KI, Durbeej M. A Family of laminin α2 chain-deficient mouse mutants: advancing the research on LAMA2-CMD. Front Mol Neurosci. 2020;13:59. doi: 10.3389/fnmol.2020.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choe SC, Hamacher-Brady A, Brady NR. Autophagy capacity and sub-mitochondrial heterogeneity shape Bnip3-induced mitophagy regulation of apoptosis. Cell Commun Signal. 2015;13:37. doi: 10.1186/s12964-015-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A. 2013;110(28):E2582–E2591. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoenherr C, Serrels B, Proby C, Cunningham DL, Findlay JE, Baillie GS, Heath JK, Frame MC. Eps8 controls Src- and FAK-dependent phenotypes in squamous carcinoma cells. J Cell Sci. 2014;127(Pt 24):5303–5316. doi: 10.1242/jcs.157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magor GW, Tallack MR, Gillinder KR, Bell CC, McCallum N, Williams B, Perkins AC. KLF1-null neonates display hydrops fetalis and a deranged erythroid transcriptome. Blood. 2015;125(15):2405–2417. doi: 10.1182/blood-2014-08-590968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hachez C, Veljanovski V, Reinhardt H, Guillaumot D, Vanhee C, Chaumont F, Batoko H. The Arabidopsis abiotic stress-induced TSPO-related protein reduces cell-surface expression of the aquaporin PIP2;7 through protein-protein interactions and autophagic degradation. Plant Cell. 2014;26(12):4974–4990. doi: 10.1105/tpc.114.134080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plissonnier ML, Lahlali T, Raab M, Michelet M, Romero-López C, Rivoire M, Strebhardt K, Durantel D, Levrero M, Mehlen P, et al. Reciprocal antagonism between the netrin-1 receptor uncoordinated-phenotype-5A (UNC5A) and the hepatitis C virus. Oncogene. 2017;36(48):6712–6724. doi: 10.1038/onc.2017.271. [DOI] [PubMed] [Google Scholar]
- 89.Li W, Zhang S, Liu J, Liu Y, Liang Q. Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and mineralization through autophagy induction. Mol Med Rep. 2019;19(5):3676–3684. doi: 10.3892/mmr.2019.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cinque L, Forrester A, Bartolomeo R, Svelto M, Venditti R, Montefusco S, Polishchuk E, Nusco E, Rossi A, Medina DL, et al. FGF signalling regulates bone growth through autophagy. Nature. 2015;528(7581):272–275. doi: 10.1038/nature16063. [DOI] [PubMed] [Google Scholar]
- 91.Hegner B, Schaub T, Janke D, Zickler D, Lange C, Girndt M, Jankowski J, Schindler R, Dragun D. Targeting proinflammatory cytokines ameliorates calcifying phenotype conversion of vascular progenitors under uremic conditions in vitro. Sci Rep. 2018;8(1):12087. doi: 10.1038/s41598-018-30626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carracedo M, Persson O, Saliba-Gustafsson P, Artiach G, Ehrenborg E, Eriksson P, Franco-Cereceda A, Bäck M. Upregulated autophagy in calcific aortic valve stenosis confers protection of valvular interstitial cells. Int J Mol Sci. 2019;20(6):1486. doi: 10.3390/ijms20061486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng XS, Meng X, Venardos N, Song R, Yamanaka K, Fullerton D, Jaggers J. Autophagy negatively regulates pro-osteogenic activity in human aortic valve interstitial cells. J Surg Res. 2017;218:285–291. doi: 10.1016/j.jss.2017.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schaub T, Gürgen D, Maus D, Lange C, Tarabykin V, Dragun D, Hegner B. mTORC1 and mTORC2 differentially regulate cell fate programs to coordinate osteoblastic differentiation in mesenchymal stromal cells. Sci Rep. 2019;9(1):20071. doi: 10.1038/s41598-019-56237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu L, Xu K, Wu Z, Chen Z, He Y, Ma C, Moqbel SAA, Ran J, Zhang C, Wu L, et al. Pioglitazone attenuates advanced glycation end products-induced apoptosis and calcification by modulating autophagy in tendon-derived stem cells. J Cell Mol Med. 2020;24(3):2240–2251. doi: 10.1111/jcmm.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen NX, O'Neill KD, Moe SM. Matrix vesicles induce calcification of recipient vascular smooth muscle cells through multiple signaling pathways. Kidney Int. 2018;93(2):343–354. doi: 10.1016/j.kint.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bakhshian Nik A, Hutcheson JD, Aikawa E. Extracellular vesicles as mediators of cardiovascular calcification. Front Cardiovasc Med. 2017;4:78. doi: 10.3389/fcvm.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zazzeroni L, Faggioli G, Pasquinelli G. Mechanisms of Arterial Calcification: The Role of Matrix Vesicles. Eur J Vasc Endovasc Surg. 2018;55(3):425–32. 10.1016/j.ejvs.2017.12.009. [DOI] [PubMed]
- 99.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 100.Cuomo F, Altucci L, Cobellis G. Autophagy Function and Dysfunction: Potential Drugs as Anti-Cancer Therapy. Cancers. 2019;11:1465. doi: 10.3390/cancers11101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chude CI, Amaravadi RK. Targeting Autophagy in Cancer: Update on Clinical Trials and Novel Inhibitors. International Journal of Molecular Sciences. 2017;18:1279. doi: 10.3390/ijms18061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ávalos Y, Canales J, Bravo-Sagua R, Criollo A, Lavandero S, Andrew F. G. Quest. Tumor Suppression and Promotion by Autophagy. BioMed Research International. 2014; 603980–95. 10.1155/2014/603980. [DOI] [PMC free article] [PubMed]
- 103.Raggi P, Bellasi A, Bushinsky D, Bover J, Rodriguez M, Ketteler M, Sinha S, Salcedo C, Gillotti K, Chertow GM. Slowing Progression of Cardiovascular Calcification With SNF472 in Patients on Hemodialysis. Circulation. 2020;141:728–39. doi: 10.1161/CIRCULATIONAHA.119.044195. [DOI] [PubMed] [Google Scholar]
- 104.Hedayati SS. A Novel Treatment for Vascular Calcification in Patients With Dialysis-Dependent Chronic Kidney Disease. Circulation. 2020;141:740–742. doi: 10.1161/CIRCULATIONAHA.119.044801. [DOI] [PubMed] [Google Scholar]
- 105.Anekonda TS, Wadsworth TL, Sabin R, Frahler K, Harris C, Petriko B, Ralle M, Woltjer R, Quinn JF. Phytic acid as a potential treatment for Alzheimer's pathology: evidence from animal and in vitro models. J Alzheimers Dis. 2011;23(1):21–35. doi: 10.3233/JAD-2010-101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available upon request to corresponding author.