FIGURE 2.
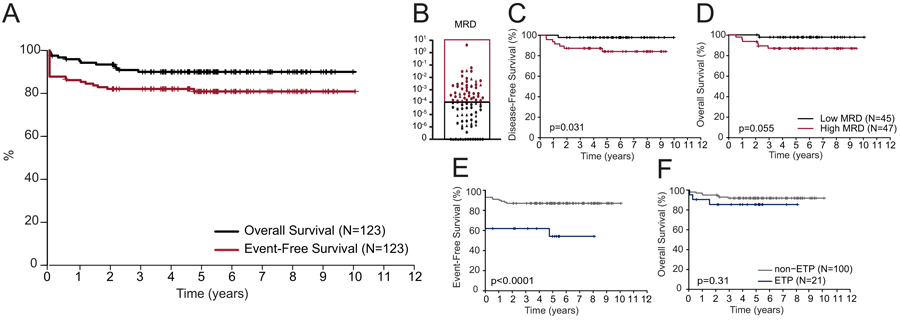
Outcomes for patients with T-ALL treated on DFCI 05-001 and 11-001. (A) Kaplan-Meier analysis of overall (OS) and event-free (EFS) survival for all 123 patients with T-ALL treated on DFCI 05-001 and 11-001. (B) End-induction minimal residual disease (MRD) results for T-ALL participants treated on DFCI Protocols 05-001 and 11-001. Kaplan-Meier analysis of (C) disease-free survival (DFS) and (D) OS for patients with low (<10−4) and high (≥10−4) MRD; and (E) EFS and (F) OS for ETP vs. non-ETP ALL. A two-sided log rank (Mantel-Cox) test was used to test for differences in survival between groups in panels B-F. ■ denotes MRD assessment by RT-PCR; ▲ denotes MRD assessment by NGS of Ig and TCR rearrangements. MRD, Minimal residual disease.
