Figure 2.
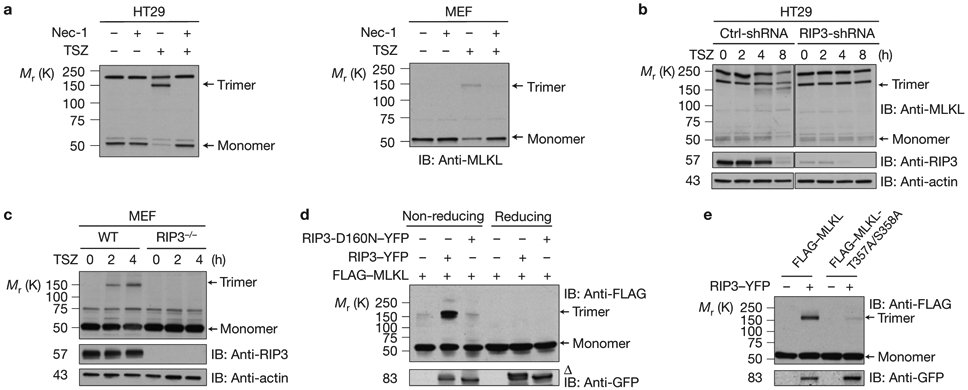
RIP3 kinase activity is critical for MLKL trimerization (a) HT29 cells (left) or MEF cells (right) were pre-treated with or without necrostatin-1 (Nec-1) and then treated with TSZ for 4 h. The cell lysates were resolved on non-reducing gel and analysed by immunoblotting with anti-MLKL antibody. (b) Control-shRNA or RIP3-shRNA HT29 cells were treated with TSZ as indicated. The cell lysates were resolved on non-reducing gel and analysed by immunoblotting with anti-MLKL antibody. (c) WT or RIP3-deficient MEF cells were treated with TSZ and cell lysates were resolved on non-reducing gel and analysed by immunoblotting with the indicated antibodies. (d) HEK293 cells were transfected with FLAG–MLKL, RIP3–YFP or RIP3-D160N–YFP as indicated. The cell lysates were resolved either on reducing or non-reducing gel and analysed by immunoblotting with the indicated antibodies. (e) HEK293 cells were transfected with FLAG–MLKL or FLAG–MLKL-T357A/S358A with or without RIP3–YFP as indicated. The cell lysates were resolved on non-reducing gel and analysed by immunoblotting with the indicated antibodies. Δ indicates phosphorylated RIP3. All western data are representative of two or three independent experiments. Uncropped images of western blots are shown in Supplementary Fig. 8.
