FIG. 6.
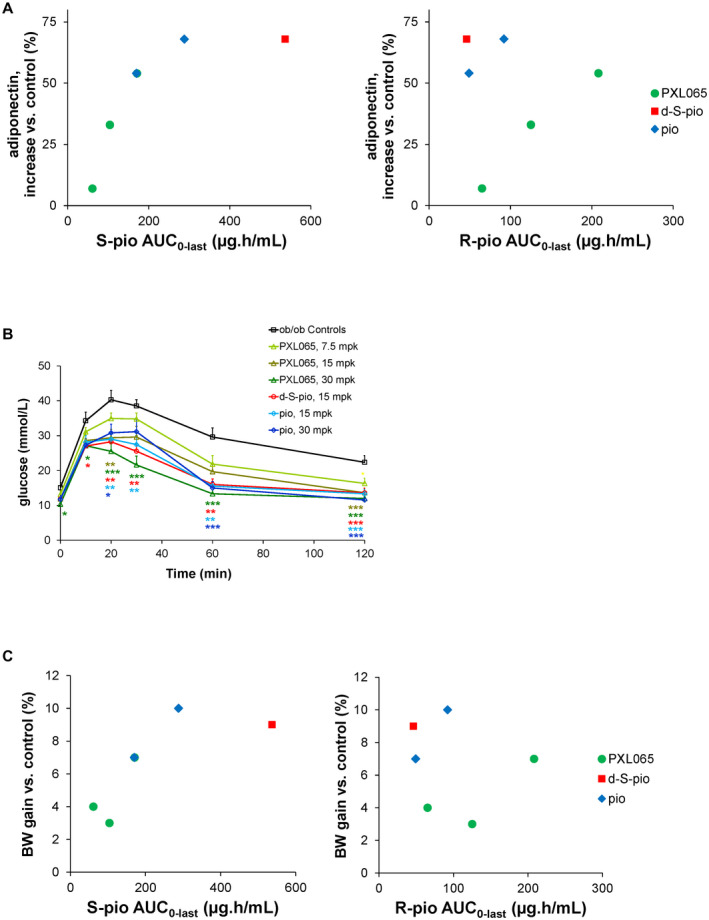
Pharmacological effect of pioglitazone (15 and 30 mg/kg), PXL065 (7.5, 15, and 30 mg/kg), and d‐S‐pio (15 mg/kg) after 4 weeks of once‐daily dosing by oral gavage in the ob/ob mouse model on plasma adiponectin (ratio high molecular weight to total) as percentage increase over ob/ob controls, as a function of exposure to S‐pio (sum of protonated and deuterated (S)‐pioglitazone, left) and R‐pio (sum of protonated and deuterated (R)‐pioglitazone, right) (A) and plasma glucose in an oral glucose tolerance test (B). Oral glucose tolerance test and analysis of variance with Dunnett’s post hoc test versus ob/ob controls: *P < 0.05, **P < 0.01, ***P < 0.001. (C) Body‐weight gain as percentage increase over ob/ob controls as function of exposure to S‐pio (left) and R‐pio (right). Abbreviations: AUC, area under the curve: and BW, body weight.
