Figure 4.
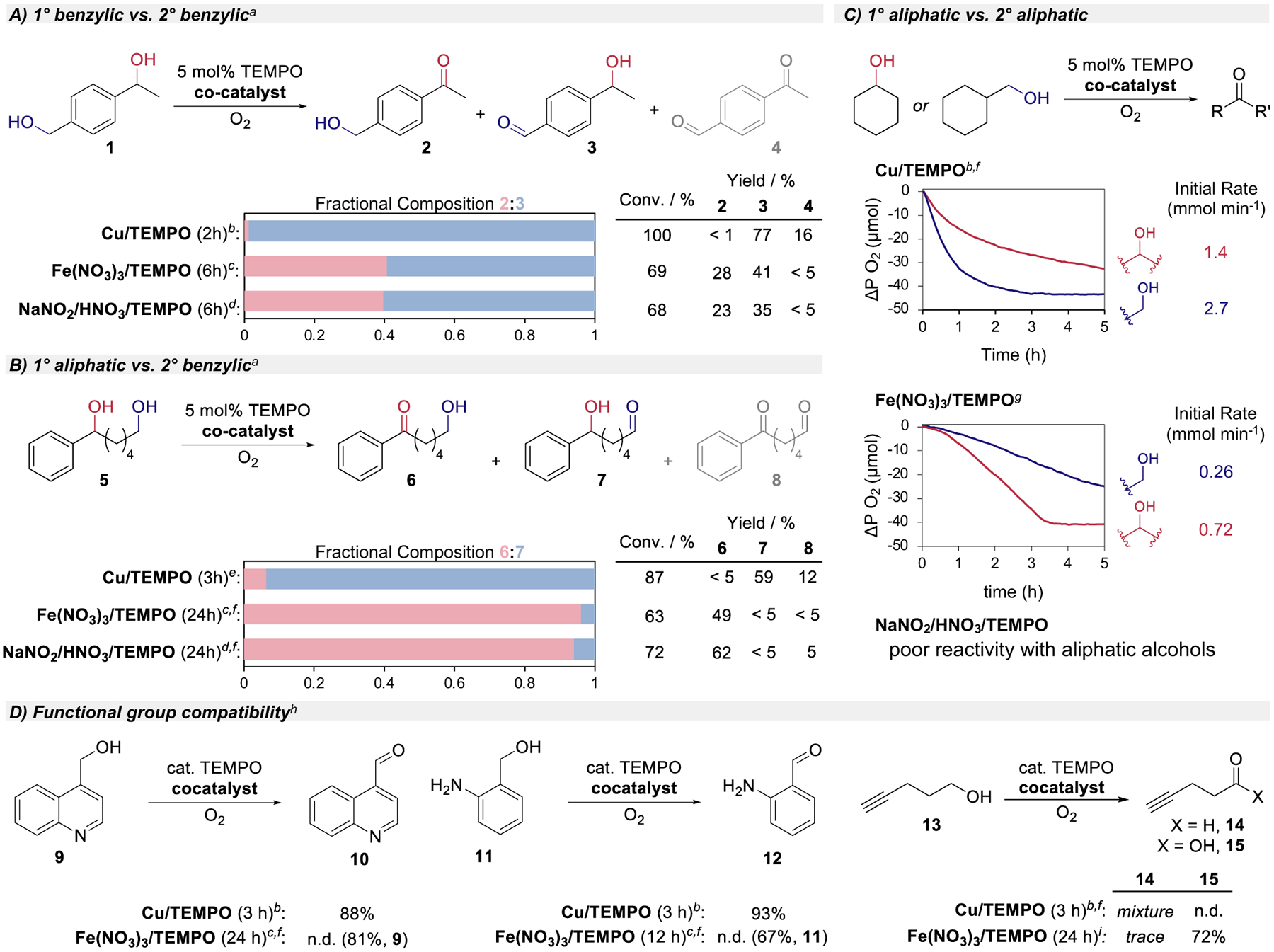
Comparison of chemoselectivity and functional compatibility with different aminoxyl cocatalyst systems for aerobic alcohol oxidation: A) intramolecular competition between 1° and 2° benzylic alcohols, B) intramolecular competition between 1° aliphatic and 2° benzylic alcohols, C) independent rate comparison of cyclohexanol and cyclohexanemethanol (for Cu/TEMPO, initial rates determined from the first 20 min; for Fe(NO3)3, rates determined from 40–60 min, i.e., after induction period), D) additional substrate probes. Conditions: rt, 5 mol% TEMPO unless noted otherwise. (A), (B), (C): 0.1 mmol substrate (0.1 M), (D): 1.0 mmol substrate (0.2 M). aRatios, yields determined by 1H NMR spectroscopy. b5 mol% [Cu(CH3CN)4]BF4, 5 mol% bpy, 10 mol% N-methylimidazole (NMI), CH3CN, air. c5 mol% Fe(NO3)3, CH3CN, air. d10 mol% NaNO2, 20 mol% HNO3, CH3CN, air. e5 mol% CuBr, 5 mol% bpy, 10 mol% NMI, CH3CN, air. f1 atm O2 rather than ambient air. g5 mol% Fe(NO3)3, 5 mol% KC1, 1,2-dichloroethane, 1 atm O2. hIsolated yields. i10 mol% TEMPO, 10 mol% Fe(NO3)3, 10 mol% KC1, 1,2-dichloroethane, 1 atm O2.
