Figure 4. RAC knockdown enhances translation.
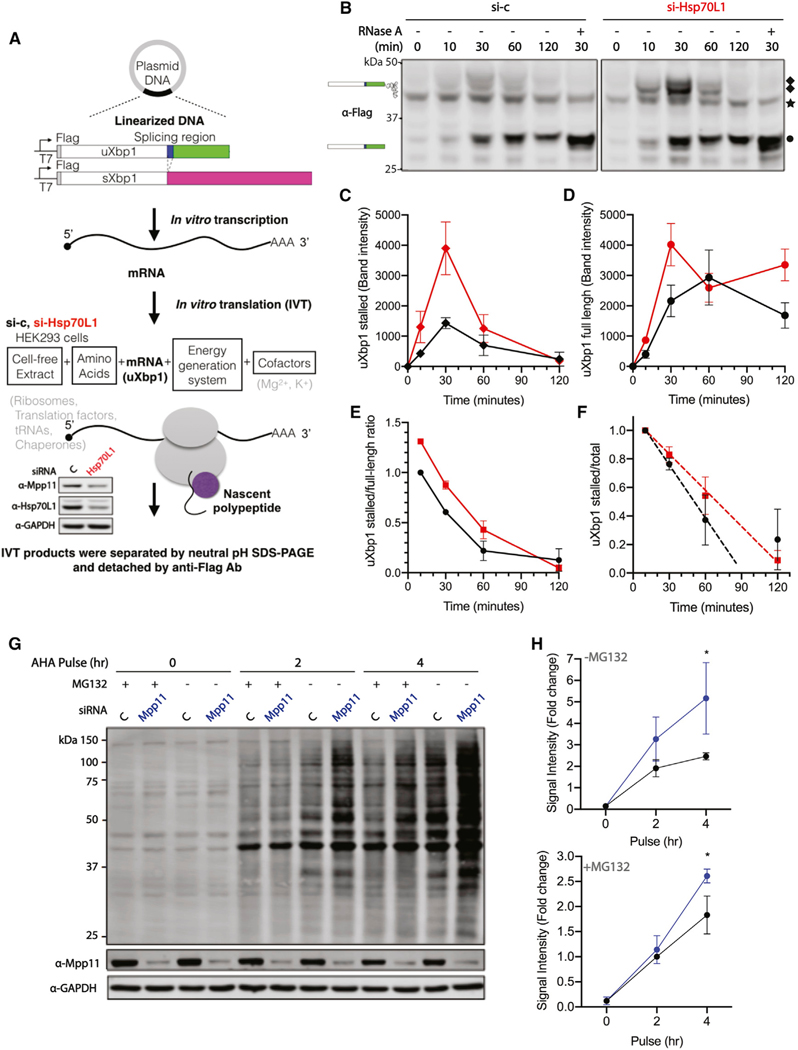
(A) Workflow of cell-free protein synthesis. The in vitro transcription plasmids were designed containing the uXbp1 or sXbp1 human open reading frame, a N-terminal Flag epitope, and 30-nt poly-A sequence after the Xbp1 stop codon. mRNA templates were synthesized from linearized DNA templates by T7 RNA polymerase and 5′ capped. In vitro translation extracts were harvested after either vehicle control (black) or Hsp70L1 (red) siRNA treatment for 48 h. The reduction of Hsp70L1 to 36% and Mpp11 to 51% of control was confirmed by western blotting (bottom). Each translation reaction contained cell-free lysate, RNA templates, amino acids mixtures, energy generation system, and cofactors.
(B) A representative western blot of in vitro translation protein products of uXbp1 mRNA is shown. The reactions were incubated at 30°C for indicated incubation time. The samples were separated on NuPAGE Bis-Tris gels, which preserve the peptidyl-tRNA ester bond, and were detected by anti-FLAG Ab. White box, the shared domain of both uXbp1 and sXbp1. Blue box, the region of the spliced intron. Green and magenta box, the distinct segment of uXbp1 and sXbp1, respectively. In each experiment, RNase A was supplemented to confirm the identity of the stalled intermediate by cleaving the peptidyl-tRNA ester bond. ◆, uXbp1 stalling intermediates; ★, non-specific band; •, uXbp1 full-length product. n = 2.
(C and D) Gels were quantified for uXbp1 stalled (C) and full-length product (D). Symbols as above. (see Figure S3A for additional biological replicates and Figure S3B for sXbp1 in vitro translation protein products.
(E) The uXbp1 stalled to full-length ratio was normalized to the value at 10 min for the vehicle si-control.
(F) The uXbp1 stalled to total product ratio as a measure of stability was normalized to the 10-min reaction of vehicle si-control or si-Hsp70L1 in the same group. Protein half-life was determined using the initial rate as reflected in the 10- and 30-min time points. Si-c t 1/2 = 42 ± 8 (mins), si-Hsp70L1 t 1/2 = 59 ± 20 (mins). (G) Pulse labeling of nascent proteins with Click-IT AHA (L-azidohomoalanine), a methionine analog, for 0, 2, and 4 h in knockdown Mpp11 HeLa cells; pretreated with or without MG132 (5 μM) for 30 min; and separated by SDS-PAGE.
(H) Quantitation of the lane intensity of the gels. n = 3.