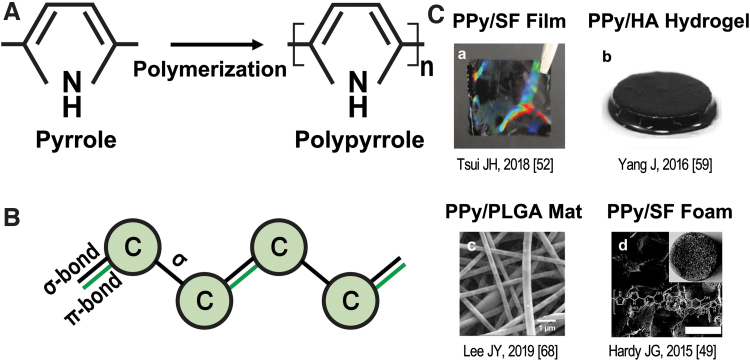
FIG. 1.
(A) Polymerization of PPy from pyrrole monomers using FeCl3 as oxidant. Oxidation of pyrrole using FeCl3: nC4H4NH + FeCl3→(C4H2NH)n + FeCl2 + HCl; oxidation (p-doping) of the PPy with dopant in the system to maintain PPy conductivity: (C4H2NH)n + xFeCl3→(C4H2NH)nCl + xFeCl2. (B) Illustration of the conjugated backbone of conductive polymer; alternating pattern of double and single bonds in the backbone. Black bond represents Sigma-bond, which strengthens the electrons; green bond represents the Pi-bond, which exists in the double bond, with lower strength. (C) Incorporation of PPy with different materials as scaffolds. (a) PPy/SF film; (b) PPy/HA hydrogel; (c) PPy/PLGA mat; (d) PPy/SF foam. FeCl3, ferric chloride; PPy, polypyrrole; SF, silk fibroin; HA, hyaluronic acid; PLGA, poly(lactic-co-glycolic acid).