Abstract
Objective
Osteoarthritis (OA) is the most common cause of chronic knee pain, often a debilitating condition that can cause a significant reduction in functional capacity. Radiofrequency is a form of neuromodulation that modulates pain signal transmission and has become progressively more common as a treatment for knee pain. This retrospective study aims to evaluate the efficacy of intraarticular radiofrequency in patients with chronic knee OA pain.
Materials and Methods
In this retrospective study, we included 129 patients undergoing intraarticular pulsed radiofrequency using the Poisson curve for energy distribution (Sluijter-Teixeira Poisson radiofrequency) (STP) from March 2018 to November 2019. Knee osteoarthritis severity was assessed prior to the procedure using the Lequesne Index, classifying patients into six groups based on level of severity. Pain intensity was assessed through a 10-cm visual analog scale (VAS), and level of patient satisfaction was assessed through a questionnaire.
Results
In the sample, pain reduction as measured by VAS compared to baseline prior to the procedure was statistically significant immediately following the procedure, at 30 days and at 90 days (p<0.001); this difference was less significant at 180 days (p<0.005). Efficacy in patients with moderate to severe disability was considerably greater than in patients with very severe to extremely severe disability. 57.36% reported that they were very satisfied, 29.46% satisfied, 9.3% neither satisfied nor dissatisfied, 2.33% dissatisfied, and 1.55% very dissatisfied.
Conclusion
Our results suggest that STP radiofrequency may be a safe and effective procedure for knee OA, able to significantly reduce VAS scores at 1 month and 3 months compared to baseline. Based on our results, a key factor to consider when treating knee OA with STP radiofrequency is that it is more effective among patients with a lower level of disability. Due to the retrospective observational study design, prospective longitudinal investigation is required to further support the recommendation of STP radiofrequency for knee OA.
Keywords: pulsed radiofrequency treatment, knee joint, osteoarthritis, knee, chronic pain
Introduction
Osteoarthritis (OA) is the most common cause of chronic knee pain; it is a debilitating condition that often causes a significant reduction in functional capacity. OA incidence is directly proportional to age, as well as presenting well-known risk factors such as gender, obesity, knee trauma, and family history.1–9 Among the pathophysiological mechanisms underlying OA, there is an imbalance between the synthesis and degradation of chondrocytes. The key to increased degradation of the chondrocytes lies in alterations of the extracellular cartilage matrix (ECM), which supports the biomechanical properties of this tissue. It has been demonstrated that factors such as IL-1, TNF, IL-6 and IL-17 are involved in the degradation process, which is fundamental in the regulation of cartilage metalloproteinases.10–12 An increase in these substances can interfere with cartilage repair mechanisms by inhibiting the response of insulin-like growth factor-1 and growth factor-β. Therefore, “anti-cytokine” therapies could potentially be successfully integrated into OA management.13–15
Standard treatments of OA include physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), tramadol, opioids, intraarticular hyaluronic acid or steroids, as well as genicular nerve ablation.16–19 In more severe cases, surgical knee arthroplasty should be considered.16 Pharmacotherapy cannot always guarantee benefits, especially in light of the high incidence of side effects. Furthermore, NSAIDs should not be administered for long periods of time due to increased risks of gastric bleeding,20 adverse cardiovascular events,21 and renal failure,22 iatrogeneses that are not favorable in the treatment of a chronic pathology. Opioids are often used, but are associated with numerous side effects, especially in the elderly. Knee surgery is not always feasible and can cause complications, such as hematomas, infections and damage to the surrounding tissue.
Pulsed and/or continuous radiofrequency are neuromodulatory and/or neurolytic techniques that represent an alternative to these therapies.23–26,39
Radiofrequency does not involve the use of drugs; it is not particularly invasive and may be repeatable.27,28 In 2011, in a double-blind randomized controlled trial, Choi et al proposed continuous radiofrequency treatment from 70 ° C to 80 ° C for 90–180 seconds on the superior lateral (SLGN), upper medial (SMGN) and lower medial (IMGN) genicular nerves (IMGN).29 The medial retinacular nerve and the infrapatellar branch of the saphenous nerve were also identified as target points.30 Similarly, in 2008, Sluijter and Teixeira reported on the successful intraarticular use of pulsed radiofrequency (PRF) using the Poisson curve for energy distribution (Sluijter-Teixeira Poisson radiofrequency) (STP).31,32 More recently, we reported on both intraarticular and genicular nerve simultaneous use with a longer period of efficacy.33,34 In that study, we carried out a retrospective analysis of patients treated with STP intra-articular knee radiofrequency over a 20-month period in a single center.
Methods
This investigation was a retrospective analysis of patient records of STP unipolar intra-articular knee radiofrequency from March 2018 to November 2019. The study was conducted at Ospedale dei Colli, Naples, and approved by the hospital’s Institutional Ethics Committee. One hundred and seventy-two consecutive patients treated with this method were included. Data from 43 patients were discarded as they were incomplete or because follow-ups did not meet the minimal number of observations. For the remaining 129 patients, data were available regarding the Lequesne Index of severity for knee osteoarthritis prior to the procedure35 and the intensity of pain using a 10-cm visual analog scale (VAS). Zero identifies no pain whatsoever and 10 identifies the most severe pain imaginable. VAS values were collected prior to the procedure (baseline), immediately following the procedure, and at 30-, 90- and 180-days post-procedure. Based on the Lequesne Index, patients were classified into six groups of differing severity of osteoarthritis.36 Medication intake before and after the procedure was evaluated, followed by further assessment after each follow-up visit. A satisfaction questionnaire was administered to all patients at 180 days, on which patients could choose between “very satisfied”, “satisfied”, “neither satisfied nor dissatisfied”, “dissatisfied” and “very dissatisfied”. An informed-consent form for non-sensitive data utilization was signed prior the procedure. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki of 1996.
Technical Procedure
Under aseptic operating room conditions, a NeuroTherm NT-1100 lesion generator was used. Following cutaneous local anesthesia with 1% lidocaine, a PRF needle (SMK C-10, 22G, active tip 10 mm; NeuroTherm, Wilmington, MA) was inserted into the joint. Insertion was performed under fluoroscopic guidance in two planes for 56 patients and under in-plane sonographic guidance for the remaining 73 patients. A superior, medial or lateral retro-patellar approach was used to enable insertion of the radiofrequency cannula as close as possible to the painful area within the joint. A “tunnel-vision” fluoroscopic technique was also adopted, taking care to visualize the intra-articular space. The lateral view is necessary to determine the depth of the needle in the joint.
Statistical Analysis
A one-way analysis of variance (ANOVA) using Microsoft Excel was used to test the statistical power of pain reduction between the different timeframes (from baseline to 180 days after treatment). In addition, t-tests were performed using Microsoft Excel between every two consecutive timeframes. A Shapiro Wilk normality test was used to detect any departure from normality for each group. P-values were corrected using the Bonferroni method and the level of significance was set at 0.05. The bar plots and linear charts were prepared using Microsoft Excel.
Results
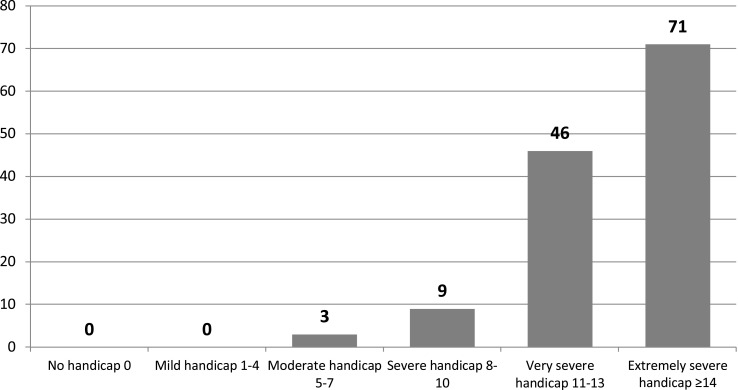
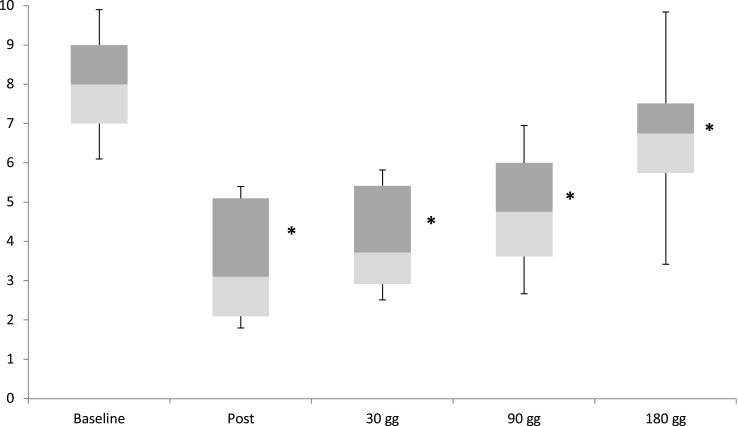
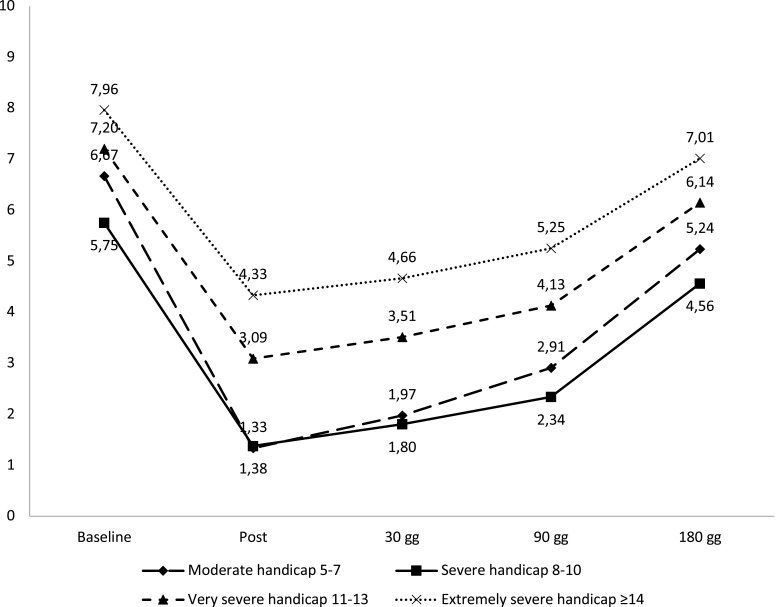
With respect to the demographic data, the 129 patients were divided as follows: 34 males, 95 females; mean age 74±10.7 years. Based on the results of the Lequesne Index of severity for osteoarthritis, the majority of patients were classified as having very severe disability in 46/129 subjects (35.66%) or extremely severe disability in 71/129 subjects (55.04%) (Figure 1). Pain reduction in terms of VAS was found to be statistically significant immediately post procedure, at 30 days and at 90 days (p<0.001); this difference was less significant at 180 days (p<0.005) (Figure 2). VAS values for first quartile, median and third quartile for all observed times are presented in Table 1. By assessing pain relief for the various disability classes obtained with the Lequesne classification, it is clear that efficacy in patients with moderate disability and severe disability was considerably more significant than in patients with very severe disability or extremely severe disability (Figure 3).
Figure 1.
Patient distribution based on the Lequesne Index of severity for osteoarthritis.
Figure 2.
Box plot of median, first and third quartile of VAS values at different observation points.
Table 1.
VAS Values of Median, First and Third Quartiles for Different Observation Points
| Baseline | After Procedure | 30d | 90d | 180d | |
|---|---|---|---|---|---|
| First quartile (min) | 7 | 2 | 2.91 | 3.62 | 5.75 |
| Median | 8 | 3 | 3.72 | 4.75 | 6.75 |
| Third quartile (max) | 9 | 5 | 5.42 | 6 | 7.51 |
Figure 3.
Pain relief related to the various disabled classes obtained using Lequesne classification.
More specifically, 2 of 3 patients in the moderate Disability group, 7 of 8 in the severe Disability group, 31 of 45 in the very Severe Disability group, and 33 of 73 in the extremely severe Disability group reported greater than 50% pain relief.
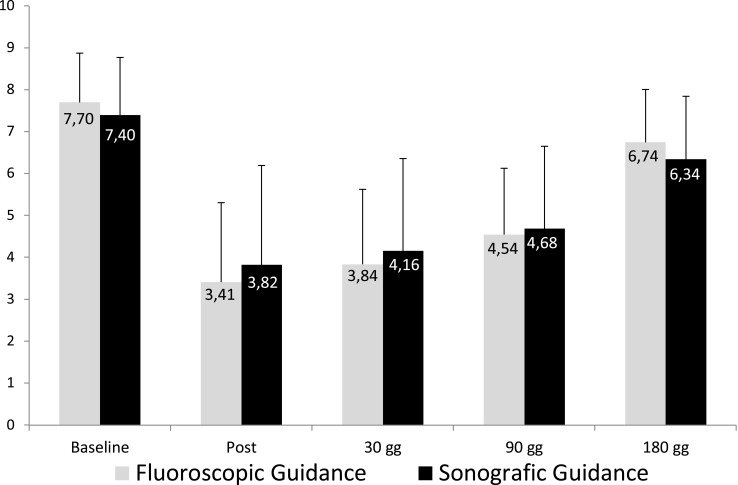
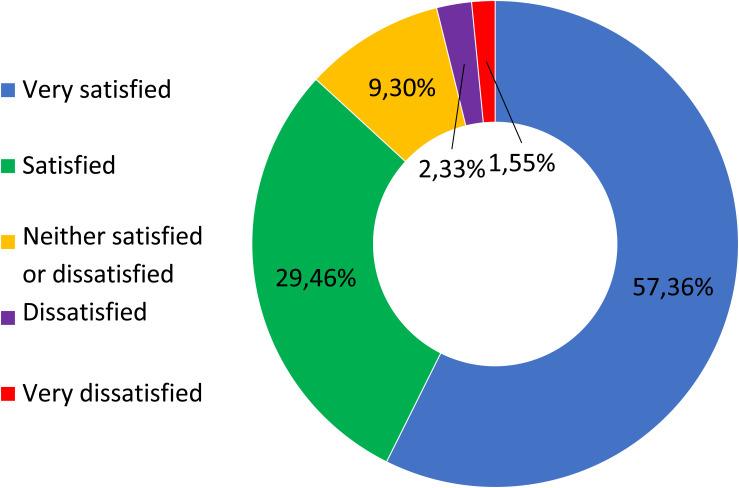
None of the 3 patients in the Moderate Disability group, none of the 8 in the Severe Disability group, 6 of 45 in the Very Severe Disability group and 21 of 73 in the Extremely Severe Disability group reported less than 30% pain relief. No difference was found in pain relief between patients treated with fluoroscopic guidance compared to those treated with ultrasound guidance (Figure 4). Likewise, regarding the degree of satisfaction at 180 days, patients declared themselves very satisfied 74/129 (57.36%), satisfied 38/129 (29.46%), neither satisfied nor dissatisfied 12/129 (9.3%), dissatisfied 3/129 (2.33%) or very dissatisfied 2/129 (1.55%) (Figure 5). At least 118/129 patients (91.47%) opined that they would repeat the procedure if necessary. No major adverse events occurred, and only three patients experienced post-procedural pain, which, in each case lasted less than 24 hours.
Figure 4.
VAS values compared between patients treated under fluoroscopic guidance vs sonographic guidance.
Figure 5.
Patients satisfaction recorded 180 days after procedure.
Discussion
Our retrospective study arose from the need to evaluate our clinical experience originating from clinical data reported in the scientific literature by Sluijter et al,30,31 and continued from an empirical evaluation of patient satisfaction data collected in our 2020 case series. Although the mechanism of action of PRF is not yet entirely clear, our data support the assertion of Schianchi et al, who postulated that intra-articular PRF may have a dual effect.37 PRF is characterized by short bursts of energy application (10–20 milliseconds), between which were interspersed long silent phases (480 milliseconds), which contribute to maintaining tissue temperature below the irreversible tissue damage threshold of 42°C. This approach suppresses excitatory C-fiber activation and the spread of pain impulse at the synaptic junction, thus creating a neuromodulatory effect. In the STP mode, radiofrequency provides a short pulse width for minimal destructive effect and a higher coefficient of variance for greater efficacy of treatment.
This pulsed method has been administered inside the intervertebral discs for discogenic pain, and with intra-articular application for arthrogenic pain, resulting in significant efficacy rates in terms of pain reduction and mobility improvement.34
The initial effect of this treatment is on nerve fibers and is thought to be due to amplification of the electric field that occurs within a closed joint. The second and most probable effect occurs due to modulation of the inflammatory response. Further to this, in a case study reported by Schianchi et al,35 the authors concluded that the biological effects of low-range electrical fields consist of a remodulation of inflammatory cytokine production. This hypothesis has been supported through in vitro investigations.37,38
The primary weakness of this study relates to its retrospective design. Even though the results were highly significant, prospective studies including control arms will be necessary in order to confirm our findings. Further, our study was performed at a single site, and generalizability of results will improve once we conduct a multi-site investigation. The minimally invasive nature and high safety levels of this procedure, in addition to the marked success rate anecdotally observed in common clinical practice, amplify the need for appropriate studies in order to clarify the efficacy of intra-articular knee PRF in OA patients.
Conclusion
In the current study, for Moderate and Severe Disability groups, intraarticular STP Pulsed Radiofrequency resulted in significantly reduced VAS scores at 1, 3 and 6 months compared to baseline in osteoarthritis pain. However, in the Extremely Severe Disability group, despite high levels of patient satisfaction, approximately one-third of patients reported less than 30% pain relief. In light of our experience, this technique should be reserved for the Moderate and Severe Disability groups as those are the groups which currently reported higher levels of satisfaction and good pain relief. In the Extremely Severe Disability group, this technique may be considered only in selected cases, when an adequate therapeutic and/or surgical alternative is not contemplated. Although this study’s results are quite encouraging, a prospective analysis will be needed in order to substantiate the relative benefits of this technique.
Disclosure
Dr Michael E. Schatman is research consultant for Firstox and Modoscript, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 2.Issa SN, Sharma L. Epidemiology of osteoarthritis: an update. Curr Rheumatol Rep. 2006;8:7–15. doi: 10.1007/s11926-006-0019-1 [DOI] [PubMed] [Google Scholar]
- 3.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Ex- amination Survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 4.Neame RL, Muir K, Doherty S, Doherty M. Genetic risk of knee osteoarthritis: a sibling study. Ann Rheum Dis. 2004;63:1022–1027. doi: 10.1136/ard.2003.014498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engström G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68:490–496. doi: 10.1136/ard.2008.089748 [DOI] [PubMed] [Google Scholar]
- 6.Felson DT. Weight and osteoarthritis. Am J Clin Nutr. 1996;63:430–432. doi: 10.1093/ajcn/63.3.430 [DOI] [PubMed] [Google Scholar]
- 7.Wilder FV, Hall BJ, Barrett JP, Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage. 2002;10:611–616. doi: 10.1053/joca.2002.0795 [DOI] [PubMed] [Google Scholar]
- 8.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45:387–398. doi: 10.3233/BIR-2008-0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113–1121. doi: 10.1001/archinternmed.2009.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malemud CJ. Fundamental pathways in osteoarthritis: an overview. Front Biosci. 1999;4:D659–D661. [DOI] [PubMed] [Google Scholar]
- 11.Haqqi TM, Anthony DD, Malemud CJ. Chondrocytes. In: Tsokos G, editor. Current Molecular Medicine: Principles of Molecular Rheumatology. Totowa (NJ): Humana Press; 2000:267–277. [Google Scholar]
- 12.Nuki G. Role of mechanical factors in the aetiology, pathogenesis and progression of osteoarthritis. In: Reginster J-Y, Pelletier JP, Martel-Pelletier J, editors. Osteoarthritis: Clinical and Experimental Aspects. Heidelberg: Springer-Verlag; 1999:101–114. [Google Scholar]
- 13.Malemud CJ, Goldberg VM. Future directions for research and treatment of osteoarthritis. Front Biosci. 1999;4:D762–D771. doi: 10.2741/A392 [DOI] [PubMed] [Google Scholar]
- 14.Chikanza I, Fernandes L. Novel strategies for the treatment of osteoarthritis. Expert Opin Investig Drugs. 2000;9:1499–1510. doi: 10.1517/13543784.9.7.1499 [DOI] [PubMed] [Google Scholar]
- 15.Malemud CJ. Cytokines as therapeutic targets for osteoarthritis. BioDrugs. 2004;18(1):23–35. doi: 10.2165/00063030-200418010-00003. PMID: 14733605. [DOI] [PubMed] [Google Scholar]
- 16.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905–1915. doi:. PMID: 11014340. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal anti-inflammatory drug or pure analgesic. J Rheumatol. 1992;19:1950–1954. [PubMed] [Google Scholar]
- 19.Rashad S, Hemingway A, Rainsford K, Revell P, Low F, Walker F. Effect of non- steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet. 1989;334:519–522. doi: 10.1016/S0140-6736(89)90651-X [DOI] [PubMed] [Google Scholar]
- 20.Marabotto E, Ziola S, Savarino V, et al. Vonoprazan fumarate for the treatment of gastric ulcers: a short review on emerging data. Clin Exp Gastroenterol. 2020;13:99–104. doi: 10.2147/CEG.S228352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang AY, Smith SM. U.S. trends in prescription nonsteroidal anti-inflammatory drug use among patients with cardiovascular disease, 1988–2016. Pharmacotherapy. 2020. doi: 10.1002/phar.2488 [DOI] [PubMed] [Google Scholar]
- 22.Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed O, Block J, Mautner K, et al. Percutaneous management of osteoarthritis in the knee: proceedings from the Society of Interventional Radiology Research Consensus Panel. J Vasc Interv Radiol. 2021;1:6. doi: 10.1016/j.jvir.2021.03.409. [DOI] [PubMed] [Google Scholar]
- 24.Filippiadis D, Charalampopoulos G, Mazioti A, et al. Interventional radiology techniques for pain reduction and mobility improvement in patients with knee osteoarthritis. Diagn Interv Imaging. 2019;100(7–8):391–400. doi: 10.1016/j.diii.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 25.Filippiadis D, Velonakis G, Mazioti A, et al. Intra-articular application of pulsed radiofrequency combined with viscosupplementation for improvement of knee osteoarthritis symptoms: a single centre prospective study. Int J Hyperthermia. 2018;34(8):1265–1269. doi: 10.1080/02656736.2017.1409910 [DOI] [PubMed] [Google Scholar]
- 26.Masala S, Fiori R, Raguso M, Morini M, Calabria E, Simonetti G. Pulse-dose radiofrequency for knee osteoartrithis. Cardiovasc Intervent Radiol. 2014;37(2):482–487. doi: 10.1007/s00270-013-0694-z [DOI] [PubMed] [Google Scholar]
- 27.Karaman H, Tufek A, Kavak GO, et al. Intra-articularly applied pulsed radiofrequency can reduce chronic knee pain in patients with osteoarthritis. J Chin Med Assoc. 2011;74:336–340. doi: 10.1016/j.jcma.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 28.Vigneri S, Sindaco G, La Grua M, et al. Electrocatheter-mediated high-voltage pulsed radiofrequency of the dorsal root ganglion in the treatment of chronic lumbosacral neuropathic pain: a randomized controlled study. Clin J Pain. 2020;36(1):25–33. doi: 10.1097/AJP.0000000000000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi WJ, Hwang SJ, Song JG, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double- blind randomized controlled trial. Pain. 2011;152(3):481–487. doi: 10.1016/j.pain.2010.09.029 [DOI] [PubMed] [Google Scholar]
- 30.Ikeuchi M, Ushida T, Izumi M, Tani T. Percutaneous radiofrequency treatment for refractory anteromedial pain of osteoarthritic knees. Pain Med. 2011;12(4):546–551. doi: 10.1111/j.1526-4637.2011.01086.x [DOI] [PubMed] [Google Scholar]
- 31.Sluijter ME, Teixeira A. Cooled radiofrequency ablation of genicular nerves for knee osteoarthritis pain: a letter to the editor. Anesth Pain Med. 2017;7(3):e46940. doi: 10.5812/aapm.46940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sluijter ME, Teixeira A, Serra V, Balogh S, Schianchi P. Intra-articular application of pulsed radiofrequency for arthrogenic pain: report of six cases. Pain Pract. 2008;8(1):57–61. doi: 10.1111/j.1533-2500.2007.00172.x [DOI] [PubMed] [Google Scholar]
- 33.Leoni MLG, Schatman ME, Demartini L, Lo Bianco G, Terranova G. Genicular nerve pulsed dose radiofrequency (PDRF) compared to intra-articular and genicular nerve PDRF in knee osteoarthritis pain: a propensity score-matched analysis. J Pain Res. 2020;13:1315–1321. doi: 10.2147/JPR.S240138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filippiadis D, Tsochatzis A, Petsatodis E, et al. Intra-articular application of sluijter-teixera poisson pulsed radiofrequency in symptomatic patients with knee osteoarthritis: focus upon clinical efficacy and safety. Pain Res Manag. 2021;2021:1–8. doi: 10.1155/2021/5554631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation–value in comparison with other assessment tests. Scand J Rheumatol. 1988;73:1. [DOI] [PubMed] [Google Scholar]
- 36.Nilsdotter A, Bremander A. Measures of hip function and symptoms: Harris Hip Score (HHS), Hip Disability and Osteoarthritis Outcome Score (HOOS), Oxford Hip Score (OHS), Lequesne Index of Severity for Osteoarthritis of the Hip (LISOH), and American Academy of Orthopedic Surgeons (AAOS) Hip and Knee Questionnaire. Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S200–S207. [DOI] [PubMed] [Google Scholar]
- 37.Schianchi PM, Sluijter ME, Balogh SE. The treatment of joint pain with intra-articular pulsed radiofrequency. Anesth Pain Med. 2013;3(2):250–255. doi: 10.5812/aapm.10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahana A, Vutskits L, Muller D. Acute differential modulation of synaptic transmission and cell survival during expo- sure to pulsed and continuous radiofrequency energy. J Pain. 2003;4(4):197–202. doi: 10.1016/S1526-5900(03)00554-6 [DOI] [PubMed] [Google Scholar]
- 39.Tun K, Cemil B, Gurcay AG, et al. Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72(5):496–500. doi: 10.1016/j.surneu.2008.11.016 [DOI] [PubMed] [Google Scholar]