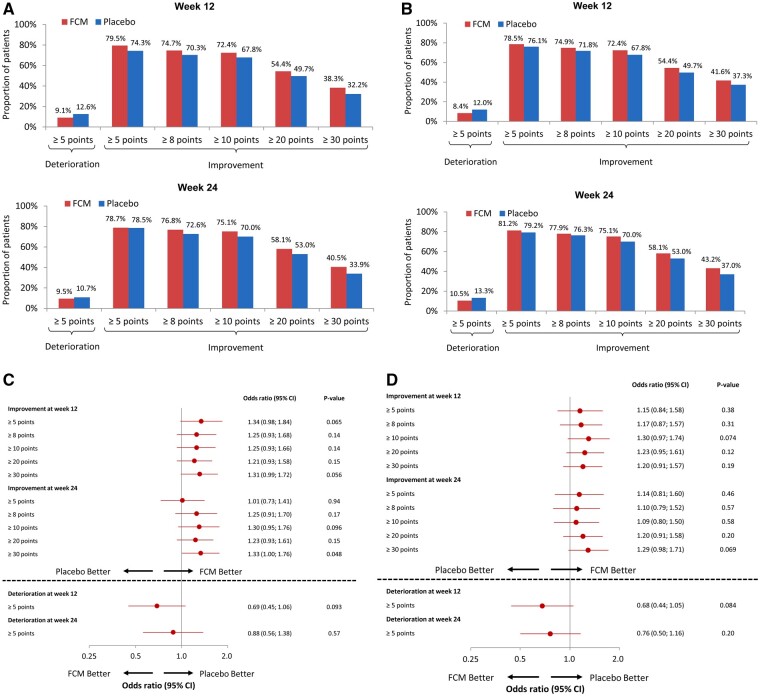
Figure 3.
Responder analyses of clinically meaningful changes in the KCCQ-12 OSS and CSS at 12 and 24 months, comparing FCM with placebo. Responder analyses of clinically meaningful changes in KCCQ-12 OSS (A and C) and KCCQ-12 CSS (B and D) at Weeks 12 and 24 after randomization. %, proportion of patients; CSS, clinical summary score; FCM, ferric carboxymaltose; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, overall summary score.