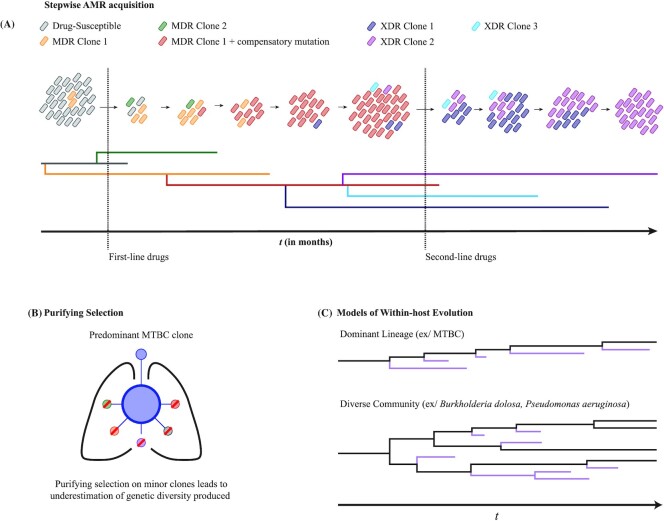
Figure 3.
Dynamics of MTBC genetic diversity within-host in the context of AMR. (A) Stepwise acquisition of chromosomal AMR mutations in the MTBC in the presence of antimicrobial pressure. Multiple AMR mutants may emerge from the same parental clonal population, and can co-exist for weeks and even months. Such population dynamics would lead to a ‘branching evolution’ pattern. Compensatory mutations that alleviate AMR mutation costs may also be acquired during the course of infection. However, a single AMR mutant clone appears to outcompete all other AMR mutant clones, and can acquire further mutations to become resistant to subsequently used antimicrobials. Phylogenetic tree used to visualize different bacterial clones over time, with colors denoting their clonality and phenotype (MDR = multidrug-resistant; XDR = extensively drug-resistant). (B) Sufficient antimicrobial pressure appears to confer strong purifying selection pressure on infecting MTBC populations. Purifying selection of minor clones would effectively lead to an underestimation of the MTBC genetic diversity that would have been produced during the course of infection, as the predominant MTBC clone would generally be the only clone sampled from a given patient. (C) Two models have been proposed for how bacterial populations evolve within-host: the Dominant Lineage model and the Diverse Community Model. The MTBC appears to follow the Dominant Lineage model, where new variants may be produced, but co-existence is transient and only one clone dominates the infection long-term. In contrast, the Diverse Community model is characterized by multiple clones maintaining the infection long-term; infections caused by the opportunistic pathogens Burkholderia dolosa and Pseudomonas aeruginosa appear to follow this model in cystic fibrosis patients (Lieberman et al. 2014; Winstanley, O'Brien and Brockhurst 2016; Clark et al. 2018). Phylogenetic tree used to visualize different bacterial clones over time; here, black clones are responsible for long-term infections, while purple clones eventually become extinct (Fig. 3B adapted from Trauner et al. 2017, with permission).