Abstract
Abstract
The epidemiology of cryptosporidiosis in southern Africa is largely unknown. The disease is associated with diarrhea and nutritional deficiencies, leading to severe morbidity and mortality among immune-compromised patients. This study aimed to assess the pooled prevalence of Cryptosporidium spp. infection among immune-compromised humans in southern Africa over the past 20 years. Reports of Cryptosporidium spp. infection in humans published between 2000 and 2020 using Google Scholar, PubMed, Ovid Medline, African Journal Online (AJOL), and Web of Science literature databases were obtained. Inclusion criteria of sorted articles for Cryptosporidium spp. infection were standardized using preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist. A total of 22 eligible studies were sorted for meta-analysis. Overall prevalence of Cryptosporidium spp. infection in southern African countries with reports was 16.8% (95% CI 9.7–25.3). Sub-group analysis showed a pooled prevalence of 25.2, 20.5, and 17.9% among HIV/AIDS patients, children, and diarrhoeic individuals, respectively. Pooled prevalence was highest in South Africa and lowest in Zimbabwe across examined individuals. The pooled prevalence of Cryptosporidium spp. infections in diarrhoeic patients was highest in individuals from Botswana (17.6%) which is significantly different (Χ2 = 9.337; P = 0.002) from South Africans (12.7%). South African individuals with HIV/AIDS showed the highest pooled prevalence of Cryptosporidium infections than other countries. The high prevalence of Cryptosporidium spp. infections among immune-compromised patients in southern Africa showed that the pathogen is of significant importance in this region. Continuous studies on the genetic characterization of Cryptosporidium spp. isolates and associated risk factors are needed across southern Africa to identify the predominant subtypes in humans.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12639-021-01436-4.
Keywords: Cryptosporidiosis, Zoonotic disease, Prevalence, HIV, Public health, Protozoa
Introduction
One of the most important neglected tropical diseases is cryptosporidiosis caused by Cryptosporidium spp. Cryptosporidiosis is primarily a water-borne disease associated with fatal diarrhea and is often reported in immune-compromised individuals. A joint Food and Agriculture Organization (FAO)/World Health Organization (WHO) expert committee has ranked Cryptosporidium spp. enteropathogen fifth among the 24 most significant foodborne parasites in a global ranking (Delahoy et al. 2018; Odeniran and Ademola 2019). Cryptosporidiosis perception among people in southern Africa is low, while the disease is exacerbated by the widespread human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), rural settlements with poor hygiene, and water shortages (Ojuromi and Ashafa 2018).
The disease is common in children, and several identified risk factors include contact with animals, malnutrition, HIV status, and water-related activities (Squire and Ryan 2017; Xiao 2010). Indicators of Cryptosporidium spp. infection include presence of watery diarrhoea, nutritional defects, elevated levels of lactoferrin and immune system related defects. Infection can be contracted through direct contact with infected humans, zoonotic (animal), and can likewise be foodborne or waterborne (Xiao 2010; Burnet et al. 2014).
Water left untreated and still used for domestic purposes such as cooking, drinking, swimming, and bathing in many rural African homes mediates the exposure to waterborne Cryptosporidium spp. (CDC 2015). Cryptosporidium spp. has been identified in animals such as poultry, fish, dogs, horses, sheep, cattle etc. (Amer et al. 2013; Bodager et al. 2015; Odeniran and Ademola, 2019). Only a few studies have been directly conducted in southern Africa on the identification of Cryptosporidium spp. species in different vertebrate hosts.
Cryptosporidium spp. enteropathogen genotypes identified in humans from Africa include, C. andersoni, C. bovis, C. canis, C. cuniculus, C. felis, C. hominis, C. meleagridis, C. muris, C. parvum, C. suis, C. ubiquitum, C. viatorum, and C. xiaoi (Squire and Ryan 2017). However, most important species identified in southern Africa were C. hominis, C. meleagridis and C. parvum. Some of the subtypes of these species are zoonotic and more studies are needed to identify the most prevalent and distributed species in humans from these regions.
To decrease the prevalence of Cryptosporidium spp. among HIV patients, the introduction of antiretrovirals have been reported to slightly restore immune function (Missaye et al. 2013; Kiros et al. 2015). Besides, HIV protease inhibitors have been suggested to serve as antiparasitic drugs in cases of cryptosporidiosis. For example, the drugs indinavir, ritonavir, and saquinavir were confirmed to have anti-cryptosporidial effects in experimental studies (Mele et al. 2003). However, their efficacy as an anti-cryptosporidial is limited and cannot be substituted as mainstream antiparasitic drugs.
The diagnosis of cryptosporidiosis in this region is mainly based on the morphological identification of Cryptosporidium spp. oocysts in faecal samples by microscopy using acid-fast stains or immunofluorescent antibody staining. More sensitive test such as polymerase chain reaction (PCR) has been limited to research and not diagnostic purposes in Africa due to the cost and expertise required.
Studies conducted on Cryptosporidium spp. infection from southern African countries have not been adequately synchronized with its associated risk factors to develop public health implications and the disease's distribution rate. Similarly, meta-analyses conducted have focused on individual studies with a regional explanation of its prevalence with many comparisons rather than improving disease awareness and providing data for policymakers.
Therefore, we investigated the prevalence, risk factors, and sub-group analyses of published data on Cryptosporidium spp. infection from humans in southern Africa. Distribution and impact of the pathogen on immune-compromised patients based on reporting was also examined in the study.
Materials and methods
Search strategy
Literature databases (PubMed, Ovid Medline, AJOL, Google Scholar, and Web of Science) were searched for published articles on Cryptosporidium spp. infection in southern Africa in the English Language from January 2000 to October 2020. The considered articles were those with full-texts. Keywords for searches include, "Cryptosporid"*, "parvum", "hominis", "species", "humans", "children", "HIV", "patients", "diarrhoea", "intestinal"*, "prevalence", "epidemiology", "South Africa", "Botswana", "Lesotho", "Malawi", "Angola", "Namibia", "Zambia", "Zimbabwe", "Mozambique", "Swaziland" and "Southern Africa". Missing articles were avoided by carefully examining the references of identified articles, while the authors independently did the extraction process to minimise error.
Inclusion and exclusion criteria
An article's inclusion criterion was primarily identified, provided the conducted study that investigated the prevalence of cryptosporidiosis in humans by a diagnostic tool was a cross-sectional type and such detected positive cases in Southern Africa. Exclusion criteria focused on study review, case reports and letters to the editor, animal studies, duplicated manuscripts, and articles with insufficient information.
Data extraction
All the authors independently examined downloaded articles to avoid bias. Articles with irrelevant objectives were removed after reading through the titles, abstracts, and full texts. Variables were generated for each manuscript and were entered in Microsoft Excel spreadsheet. Some of the variables included record information of the authors, total cases examined, the number of positive cases detected, diagnostic techniques (Microscopy, Polymerase Chain Reaction, and IFAT- indirect fluorescent antibody test), the country where the study was done, study area, age group, and gender. Other attributes were those associated with risk factors such as occupation, contact with animals, water use, frequency of use, disease pathologies etc. Cross evaluation among authors was done to resolve all forms of disagreements in computing the data.
Quality assessment
PRISMA checklist was used to standardize the inclusion criteria of all relevant information in the study analysis (Moher et al. 2010) (Supplementary file). Each article was subjected to a quality assessment scale (1–10) generated in line with the objective of the study (low quality, < 5.0; moderate quality, 5.0–7.5; and high quality, 7.5–10.0). Excluded published articles were those with lesser than acceptable qualities (> 5.0) for the analysis.
Statistical analysis
METAXL® (version 3.1) was used to perform the meta-analysis. The quality effects model was used to obtain the pooled prevalence of the study estimate. The overall prevalence of the quality effects model findings was assessed using the validity of the tests. To evaluate the heterogeneity between test results, Cochran's Q test and the inconsistency (I2) indicator were used. The Luis Frya-Kanamori (LFK) index was obtained by plotting the z-score against the double-arcsin prevalence of the publications examined. This suggests substantial asymmetry when the LFK index reaches ± 2, (publication bias) (Barendregt and Doi 2015). WINPEPI (version11.65) was used for the Pearson chi-square, while Tukey's post-hoc multiple pair-wise one-way ANOVA comparison test was used to compare disease trends among countries using GraphPad Prism (version 5). Confidence intervals was set at ninety-five percent with minimum and maximum values. A map was constructed with qGIS (version 2.8.10) to show Cryptosporidium spp. infection distribution in southern African countries.
Results
Study characteristics
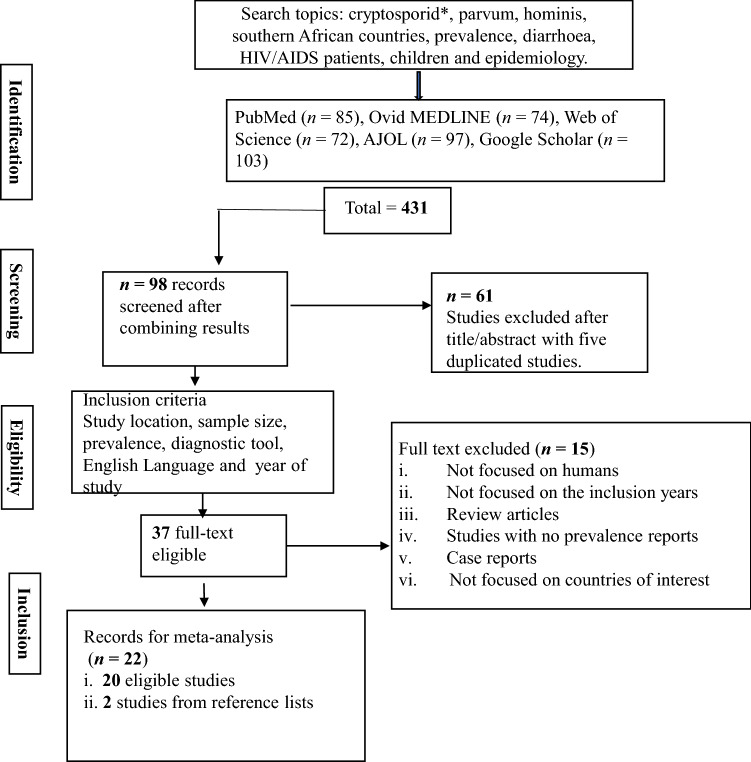
A total of 431 published articles were selected on Cryptosporidium spp. infection from the screening of five databases and reference lists of downloaded relevant studies (Fig. 1). The initial screening of study titles and abstracts led to the exclusion of 61 studies from the combined searches of 98 records. Further excluded studies due to article duplication during sorting were five studies. The remaining study were read individually by the authors and excluded 15 studies that did not meet the inclusion criteria (these were studies outside the range of selected years). Therefore, 22 studies were included in the meta-analysis (Fig. 1). Examined cases were observed in South Africa (n = 7), Botswana (n = 5), Zambia (n = 4), Malawi (n = 3), Zimbabwe (n = 2) and Angola (n = 1). It was observed that several southern African countries have not published data for human cryptosporidiosis or published in non-visible journals in recent years as observed from the database. In total, 5932 individuals were considered in the study, and the immune-compromised patients include 3959 children, 1722 diarrheic patients, 1073 HIV/AIDS seropositive individuals. A total of 589 individuals were reported as immune-competent. Four studies reported patients who were both children and HIV patients (n = 922), three studies reported patients who were both children and diarrheic (n = 1059), while a study reported patients who were diarrheic (n = 97) and positive for HIV/AIDS (n = 151).
Fig. 1.
Literature databases search on Cryptosporidium infection of humans in southern Africa
Cryptosporidium infections in southern Africa
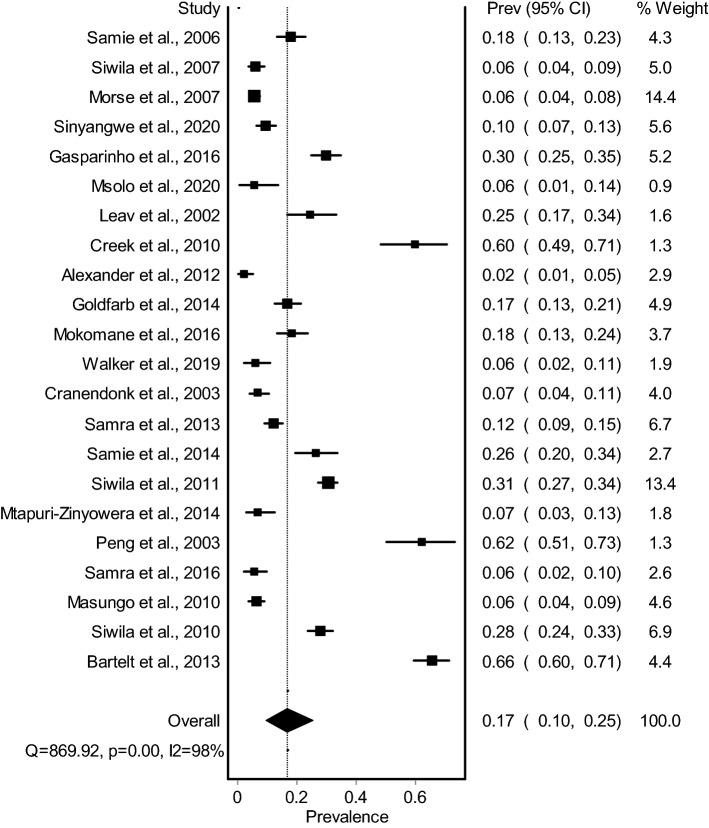
The pooled prevalence estimates of Cryptosporidium spp. spp. in humans (n = 22) with individual studies from southern Africa on human Cryptosporidium spp. infections are shown in a forest plot (Fig. 2). Studies revealed pooled prevalence of 16.8% (95% CI 9.7–25.3); I2 = 97.6; Q = 869.9; df = 21; P < 0.0001. The prevalence of Cryptosporidium spp. of each study varied from 2.4 to 65.7% (median = 14.5), with substantial heterogeneity among studies (Fig. 2). The LFK index of 1.17 showed no asymmetry, indicating that there was no bias. Based on available information from countries with cryptosporidiosis reports, the pooled prevalence was highest in South Africa with 21.8% (95% CI 6.1–42.9), and lowest in Zimbabwe 6.6% (95% CI 4.4–9.3) (Table 1). Only one study met the inclusion criteria in Angola, with prevalence of 29.9%. Statistical analysis showed that there is significant increase (Χ2 = 67.9; P < 0.0001) in the prevalence of immune-compromised patients (20.3%) compared to immune-competent individuals (6.3%) in southern Africa. The forest plot of the pooled prevalence of immune-compromised patients was reported (Fig. 3). A map showing the recent distribution of Cryptosporidium spp. infection studies across southern African countries was illustrated in Fig. 4.
Fig. 2.
Forest plot of Cryptosporidium infection among human population in southern Africa between 2000 and 2020
Table 1.
Total report of Cryptosporidium infection in humans and associated species in southern Africa between 2000 and 2020
| Sample size | No positive | Pooled Prev | 95% CI | Cochran’s Q | I2 | df | α0.05 | Chi2, OR/Anova | |
|---|---|---|---|---|---|---|---|---|---|
| Total infection | 5932 | 1121 | 16.8 | 9.7–25.3 | 869.9 | 97.6 | 21 | < 0.0001 | |
| Countries | |||||||||
| South Africa | 1385 | 339 | 21.8* | 6.1–42.6 | 289.2 | 97.9 | 6 | < 0.0001 | P < 0.0001 |
| Botswana | 849 | 142 | 13.3 | 1.8–31.1 | 119.3 | 96.6 | 4 | < 0.0001 | |
| Zambia | 1804 | 403 | 20.3* | 7.5–36.8 | 139.8 | 97.9 | 3 | < 0.0001 | |
| Malawi | 1144 | 109 | 8.5 | 0.0–100.0 | 112.5 | 99.1 | 2 | < 0.0001 | |
| Zimbabwe | 413 | 27 | 6.6 | 4.4–9.3 | 0.1 | 0.0 | 1 | 0.729 | |
| Angola | 337 | 101 | – | – | – | – | – | – | |
| Children with Cryptosporidium spp. infection | |||||||||
| Children infection | 3959 | 913 | 20.5 | 9.6–34.1 | 626.7 | 98.1 | 12 | < 0.0001 | |
| Countries | |||||||||
| South Africa | 1069 | 306 | 24.1* | 1.9–56.5 | 285.9 | 98.6 | 4 | < 0.0001 | P < 0.0001 |
| Botswana | 327 | 51 | 15.3 | 8.0–24.3 | 2.1 | 51.8 | 1 | 0.1500 | |
| Zambia | 1309 | 362 | 26.6* | 13.2–42.5 | 42.7 | 95.3 | 2 | < 0.0001 | |
| Malawi | 917 | 93 | 8.5 | 0.0–100.0 | 112.5 | 99.1 | 1 | < 0.0001 | |
| Diarrhoeic patients with Cryptosporidium spp. infection | |||||||||
| Diarrhoeic patients | 1722 | 333 | 17.9 | 9.8–27.6 | 126.9 | 93.7 | 8 | < 0.0001 | |
| Countries | |||||||||
| South Africa | 592 | 81 | 12.7 | 3.6–25.6 | 12.1 | 83.4 | 2 | 0.0020 | X2 = 9.337; P = 0.002; OR = 0.63 |
| Botswana | 685 | 138 | 17.6# | 3.5–37.9 | 75.8 | 96.0 | 3 | < 0.0001 | |
| Malawi | 108 | 13 | – | – | – | – | – | – | |
| Angola | 337 | 101 | – | – | – | – | – | – | |
| HIV/AIDS patients with Cryptosporidium spp. infection | |||||||||
| HIV/AIDS patients | 1073 | 305 | 25.2 | 5.7–51.0 | 240.5 | 96.8 | 4 | < 0.0001 | |
| Countries | |||||||||
| South Africa | 747 | 274 | 34.9 | 10.9–63.3 | 143.8 | 97.9 | 3 | < 0.0001 | |
| Zambia | 326 | 31 | – | – | – | – | – | ||
| Diagnostic technique | |||||||||
| ZN | 3676 | 568 | 13.5 | 6.1–23.1 | 332.7 | 97.3 | 9 | < 0.0001 | F2,22 P = 0.5646 R2 = 0.0506 |
| ELISA | 1501 | 413 | 24.4# | 7.8–45.8 | 318.9 | 98.1 | 6 | < 0.0001 | |
| PCR | 2486 | 313 | 11.0 | 4.0–20.5 | 181.1 | 96.1 | 7 | < 0.0001 | |
| Species in humans | |||||||||
| Cryptosporidium spp. species characterised | 123 | 3.8 | 1.3–7.2 | 100.1 | 91.0 | 9 | < 0.0001 | ||
| C. hominis | 186 | 105 | 56.5 | – | – | – | 5 | – | |
| C. parvum | 182 | 58 | 31.9 | – | – | – | 4 | – | |
| C. meleagridis | 79 | 4 | 5.1 | – | – | – | 2 | – | |
| C. andersoni | 50 | 1 | 2.0 | – | – | – | – | – | |
| C. parvum/C. hominis | 50 | 1 | 2.0 | – | – | – | – | – |
I2, level of inconsistency; df, degree of freedom; α0.05, level of significance; OR, odd ratio; F, variance between and within groups; R, coefficient of determination; CI, confidence interval; HIV+, human immunodeficiency virus positive; HIV−, human immunodeficiency virus negative; *, shows significance at P < 0.05; #, no significance at P > 0.05
Fig. 3.
Forest plot of Cryptosporidium infection among immune-compromised patients. A. Cryptosporidium infection in children; B. Cryptosporidium infection in diarrhoeic patients; C. Cryptosporidium infection in HIV/AIDS patients
Fig. 4.
Map showing southern African countries and the intensity of Cryptosporidium infection studies reported in the literature between 2000 and 2020
Pooled prevalence and heterogeneity of Cryptosporidium infections in children
Substantial heterogeneity was observed across studies on Cryptosporidium spp. infections in children. The pooled prevalence and heterogeneity variables revealed 20.5% (95% CI 9.6–34.1); I2 = 98.1; Q = 626.7; P < 0.0001. The LFK (0.02) indicates that there is no asymmetry. Cryptosporidiosis pooled prevalence showed that children in Zambia were most vulnerable with 26.6% (95% CI 13.2–42.5), followed by South African children with 24.1% (95% CI 1.9–56.5), and least in Malawian children 8.5% (95% CI 0.0–100) (Table 1). Considering the latter pooled prevalence, the confidence interval showed that the prevalence could rise further depending on the number of studies conducted across the country (Fig. 3a).
Heterogeneity of Cryptosporidium infections in diarrhoeic patients
A total of 1722 individuals with diarrhea were examined for cryptosporidial infections, of which 333 were reported positive. It was observed that most examined studies (98.3%) with diarrhoeic patients were children. Reported studies were from Botswana, South Africa, Malawi, and Angola. The pooled prevalence and heterogeneity variables of diarrhoeic patients with cryptosporidiosis showed 17.9% (9.8–27.6); I2 = 93.7; Q = 126.9; df = 8; P < 0.0001 as observed from the forest plot (Fig. 3b). Most studies were retrieved from Botswana, followed by South Africa. The pooled prevalence was highest in Botswana (17.6%) but not significantly different (Χ2 = 0.357; P = 0.550) from South Africa (12.7%) (Table 1).
Pooled prevalence of Cryptosporidium infections in HIV/AIDS patients in southern Africa
A total of 305 individuals positive with HIV/AIDS were observed to test positive to Cryptosporidium spp. infection out of examined 1073 individuals. The LFK index of 0.59 indicates that there is no asymmetry in the analysed studies. There is substantial heterogeneity with pooled prevalence showing 25.2% (5.7–51.0); I2 = 98.3; Q = 240.5; P < 0.0001 (Fig. 3c). Studies were only reported in South Africa and Zambia (Table 1). The prevalence in South Africa (34.9%) was significantly higher (X2 = 81.024; P < 0.0001) than the study from Zambia (9.5%). The presence of lactoferrin was 59.1% in Cryptosporidium spp.-positive patients observed in a study.
Diagnostic techniques
Studies were mostly examined with light microscopy using Ziehl–Neelsen staining techniques. The pooled prevalence based on light microscopy, immunoassay (IFAT, ELISA) and PCR methods was 13.5% (6.1–23.1), 24.4% (7.8–45.8) and 11.0% (4.0–20.5), respectively (Table 1). Tukey post-hoc multiple comparison test showed no significant difference (P = 0.5646; R2 = 0.0506) between the three diagnostic techniques. Five countries (Zambia, Malawi, Botswana, South Africa, and Zimbabwe) reported studies using light microscopy and immunoassay, respectively. For the PCR technique, studies were observed in four countries with most reports from South Africa (n = 3), while Malawi and Botswana (n = 2) followed, and least in Zambia (n = 1).
Species variability in southern Africa
A total of eight studies characterized Cryptosporidium spp. subtypes with PCR. The characterized Cryptosporidium spp. species showed that C. hominis was mostly observed with 56.5% distribution, followed by C. parvum with 31.9%. Cryptosporidium spp. hominis sequences analysis with either HSP70 and GP60 genes showed the presence of five sub-families (Ia, Ib, Id, Ie and If) across all available studies. The sequence analysis of GP60 genes of C. parvum showed three sub-families (IIb, IIc and IIe) across examined studies. Mixed infection of C. parvum and C. hominis showed a 2.0% distribution of the examined studies (Table 1). Only one sub-family (IId) was identified for C. meleagridis (Table 2). Of the reviewed studies, C. meleagridis showed 5.1% distribution, while C. andersoni stands at 2.0%.
Table 2.
Reported genotypes of C. hominis, C. parvum and C. meleagridis in southern Africa from molecular characterisation and sequencing
| Species/genotype | Host | Percentage positives | Subfamily | Subtype | References |
|---|---|---|---|---|---|
| Total | Country | ||||
| C. hominis | Malawi | 63.6 | Ia, Ib, Id, Ie | Peng et al. (2003) | |
| 95.3 | Morse et al. (2007) | ||||
| 58.1 | Gatei et al. (2003) | ||||
| South Africa | 75.0 | Ib, Ie | IbA12G3R2; IbA10G2; IeA11G3T3 | Samra et al. (2016) | |
| 76.0 | Ia, Ib, Id, Ie, If | IaA20R3; IaA25G1R3; IaA17R3; IbA9G3; IbA10G1; IdA20; IdA25; IdA26; IdA24; IeA11G3T3b; IfA14G1; IfA12G1 | Samra et al. (2013) | ||
| 75.0 | Ia, Ib, Id, Ie | Leav et al. (2002) | |||
| 20.0 | Siwila et al. (2007) | ||||
| 81.8 | Samie et al. (2006) | ||||
| Botswana | 41.0 | Creek et al. (2010) | |||
| C. parvum | Malawi | IIc, IIe | Peng et al. (2003) | ||
| South Africa | 20.0 | IIb, IIc, IIe | IIbA11; IIcA5G3bb; IIeA12G1 | Samra et al. (2013) | |
| 25.0 | IIc | Leav et al. (2002) | |||
| 80.0 | Siwila et al. (2007) | ||||
| 18.2 | Samie et al. (2006) | ||||
| Botswana | 50.0 | Creek et al. (2010) | |||
| C. meleagridis | Malawi | 4.7 | Morse et al. (2007) | ||
| South Africa | 4.0 | IId | IIIdA4 | Samra et al. (2013) |
Sensitivity test and limitations
The stability and reliability of the analysed results were examined from the sensitivity tests of individual data analysed in the METAXL. The analysis from the funnel plot within the 95% confidence interval and doi plot showed LFK index of 1.17 on the overall analysis, which ruled out significant bias risk of the analysed studies on human cryptosporidiosis in southern Africa. The LFK indexes of sub-group analysis have been reported in each sub-section of the result. In cases of publication bias, there is major asymmetry that could result from few cases examined in sub-group assessment.
Discussion
Cryptosporidiosis is a disease of the immune-compromised individuals, and it is often endemic in areas with poor social infrastructures such as lack of safe drinking water and sanitation problems. Studies on Cryptosporidium spp. infection in southern Africa within the past 20 years have been scanty, despite the burden of cryptosporidiosis and its associated risk factors. Southern Africa is disproportionately affected by cryptosporidiosis. This may be due to the fact that approximately 70% of its population live with HIV/AIDS, 31% of newly infected individuals with HIV, and 34% of individuals dying from AIDS are in the region (Ojuromi and Ashafa 2018; WHO 2015). South Africa has the largest population living with HIV in Southern Africa (CIA 2016).
The result from this meta-analysis showed that the overall prevalence of 16.8% Cryptosporidium spp. infection among examined humans is high in southern Africa. The published articles showed that perception and awareness of the pathogen are low. The high number of immune-compromised individuals could be correlated with the high prevalence of Cryptosporidium spp. infection. For instance, the prevalence rate of HIV individuals was estimated between 1.0 and 26.5% in this region, while adult HIV prevalence exceeded 20% in Lesotho, Swaziland and Botswana. This is higher than pooled prevalence of the disease in HIV/AIDS patients from Nigeria (Karshima and Karshima 2020), and within the global range of prevalence in sub-Saharan Africa (Aldeyarbi et al. 2016). Cryptosporidium spp. infection was highest in South Africa compared to other pooled studies from other countries from this study. This could be associated with the high population of immune-compromised individuals within the country.
Notably, Cryptosporidium spp. infection was highest in children less than five years old. Meanwhile, children are disproportionately affected by cryptosporidiosis as observed in this study, with highest pooled prevalence in Zambia. The watery diarrhoea accompanied by dehydration and weakness, could lead to death easily. This could contribute significantly to the globally estimated 800,000 deaths in children due to cryptosporidiosis annually (Sow et al. 2016; Odeniran and Ademola 2019).
The high prevalence of Cryptosporidium spp. infection observed in diarrhoeic patients, especially studies from Botswana and South Africa, could be linked to the anthroponotic nature of some Cryptosporidium spp. subtypes and hygiene. For instance, Cryptosporidium spp. oocysts and isolates have been reported to be present in the surface waters of the Vaal Dam, treated effluents, drinking water in South Africa (Dungeni and Momba 2010), and piped-water in Zambia (Nchito et al 1998). The burden of diarrhea is particularly high in children, which could be linked with water-borne cryptosporidiosis. This protozoon is the second cause of severe diarrhea and the leading cause of death in children (Kotloff et al. 2013; Sow et al. 2016). The elevated lactoferrin level observed in Cryptosporidium spp. positive individuals in a study (Samie et al. 2016), indicated that inflammation is likely present. However, more studies are needed to correlate elevated lactoferrin and cryptosporidiosis in immune-compromised patients.
Low sensitivity diagnostic techniques are major problems in southern African countries. Even though molecular studies were done, genetic characterization of Cryptosporidium spp. species is lacking, which has limited our knowledge of Cryptosporidium spp. species and subtypes across the region. The high prevalence observed from studies examined with immunoassay, could be due to circulating IgG antibodies or sensitivity of the rapid diagnostic kits used for diagnosis.
Most characterized species with GP60 genes revealed C. hominis followed by C. parvum across southern Africa in this study, which is similar to an earlier study on Africa (Squire and Ryan 2017). Moreover, C. hominis and anthroponotic C. parvum subtypes isolated revealed both zoonotic and anthroponotic transmission in southern Africa. Earlier, there have been reports of C. hominis subtypes dominance infecting humans in several studies from Africa, regardless of the immune status (Maikai et al. 2012; Helmy et al. 2013; Aldeyarbi et al. 2016). Although only a study from Zambia reported an animal contact rate of 11.4% (Sinyangwe et al. 2020), the association between human and animal with diarrhea, could be responsible for the increasing zoonotic danger of C. parvum isolates (Siwila et al. 2007), particularly among the immune-compromised patients in southern Africa. Some C. parvum subtypes have been reported to be human-adapted subtypes, that could be transmitted from person to person, with origins from humans (Morse et al. 2007). The reporting of C. meleagridis in this study is not surprising, as the African population has a growing immune-compromised population with a previous report of 21% in these populations prone to infection by this pathogen (Morgan et al. 2000; Ben Abda et al. 2011; Aldeyarbi et al. 2016). Cryptosporidial infection was observed to be highest among HIV/AIDS patients, followed by children and then diarrhoeic individuals. The pooled prevalence across these groups was higher than 20%, which indicates that infection is more likely within the immune-compromised patients.
Recent studies showed that the absence of reports for four countries (Lesotho, Namibia, Mozambique, and Swaziland) could be due to low awareness of the importance of cryptosporidiosis or local publication of Cryptosporidium spp. infection reports (Fig. 4).
Conclusions
Cryptosporidium spp. infection was highest in HIV/AIDS patients (25.2%), followed by children (20.2%) and diarrheic patients (17.9%). Although, there is no significant difference in the prevalence. These categories of individuals could be at risk of infection if prompt awareness and treatment are neglected. Areas with a high density of livestock and the presence of humans with Cryptosporidium spp. infection, especially immune-compromised individuals, could mean that routine screening for opportunistic infections should be prioritized to avoid mortalities and spread of infection. More studies are needed to be conducted in southern Africa, such as correlating the significance of highly active antiretroviral therapy (HAART) positive or negative individuals and evaluating the CD4+ cell counts with Cryptosporidium spp. infections. Awareness on the zoonotic and anthroponotic nature of several Cryptosporidium spp. subtypes is needed, while Cryptosporidium spp. screening should be included in HIV/AIDS screening procedures in southern Africa.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors appreciate University of KwaZulu Natal, Durban, South Africa, for access into their literature databases.
Funding
There is no funding for this study.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree to publish the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aldeyarbi HM, Abu El-Ezz NMT, Karanis P. Cryptosporidium and cryptosporidiosis: the African perspective. Environ Sci Pollut Res. 2016;23(14):13811–13821. doi: 10.1007/s11356-016-6746-6. [DOI] [PubMed] [Google Scholar]
- Alexander KA, Herbein J, Zajac A. The occurrence of Cryptosporidium and Giardia infections among patients reporting diarrheal disease in Chobe District, Botswana. Adv Infect Dis. 2012;2:143–147. doi: 10.4236/aid.2012.24023. [DOI] [Google Scholar]
- Amer S, Zidan S, Feng Y, Adamu H, Li N, Xiao L. Identity and public health potential of Cryptosporidium spp. in water buffalo calves in Egypt. Vet Parasitol. 2013;191:123–127. doi: 10.1016/j.vetpar.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Barendregt JJ, Doi SA (2015) MetaXL User Guide Version 3.1, Epigear International Pty Ltd, Queensland. www.epigear.com
- Bartelt LA, Sevilleja JE, Barrett LJ, Warren CA, Guerrant RL, Bessong PO, et al. High anti-Cryptosporidium parvum IgG seroprevalence in HIV-infected adults in Limpopo, South Africa. Am J Trop Med Hyg. 2013;89:531–534. doi: 10.4269/ajtmh.12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abda I, Essid R, Mellouli F, Aoun K, Bejaoui M, Bouratbine A. Cryptosporidium infection in patients with major histocompatibility complex class II deficiency syndrome in Tunisia: description of five cases. Arch Pediatr. 2011;18:939–944. doi: 10.1016/j.arcped.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Bodager JR, Parsons MB, Wright PC, Rasambainarivo F, Roellig D, Xiao L, et al. Complex epidemiology and zoonotic potential for Cryptosporidium suis in rural Madagascar. Vet Parasitol. 2015;207(1):140–143. doi: 10.1016/j.vetpar.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Burnet JB, Penny C, Ogorzaly L, Cauchie HM. Spatial and temporal distribution of Cryptosporidium and Giardia in a drinking water resource: implications for monitoring and risk assessment. Sci Total Environ. 2014;472:1023–1035. doi: 10.1016/j.scitotenv.2013.10.083. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015) Domestic water, sanitation, and hygiene epidemiology, Atlanta, USA. http://www.cdc.gov/ncezid/dfwed/waterborne/global.html. Accessed 25 Sept 2016
- CIA (2016) Central Intelligence Agency. World Fact Book; 2016. https://www.cia.gov/library/publications/the-world-factbook/rankorder/2156rank.html
- Cranendonk RJ, Kodde CJ, Chipeta D, Zijlstra EE, Sluiters JF. Cryptosporidium parvum and Isospora belli infections among patients with and without diarrhea. East Afr Med J. 2003;80:398–401. [PubMed] [Google Scholar]
- Creek TL, Kim A, Lu L, Bowen A, Masunge J, Arvelo W, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2010;53:14–19. doi: 10.1097/qai.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- Delahoy MJ, Omore R, Ayers TL, Schilling KA, Blackstock AJ, Ochieng JB, Moke F, Jaron P, Awuor A, Okonji C, et al. Clinical, environmental, and behavioral characteristics associated with Cryptosporidium infection among children with moderate-to-severe diarrhea in rural western Kenya 2008–2012: the Global Enteric Multicenter Study (GEMS) PLoS Negl Trop Dis. 2018;12:e0006640. doi: 10.1371/journal.pntd.0006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungeni M, Momba M. The abundance of Cryptosporidium and Giardia spp. in treated effluents produced by four wastewater treatment plants in the Gauteng Province of South Africa. Water SA. 2010;36:425–431. doi: 10.4314/wsa.v36i4.58413. [DOI] [Google Scholar]
- Gasparinho C, Mirante MC, Centeno-Lima S, Istrate C, Mayer AC, Tavira L, Nery SV, Brito M. Etiology of diarrhea in children younger than 5 years attending the Bengo General Hospital in Angola. Pediatr Infect Dis J. 2016;35(2):e28–e34. doi: 10.1097/INF.0000000000000957. [DOI] [PubMed] [Google Scholar]
- Gatei W, Greensill J, Ashford RW, Cuevas LE, Parry CM, Cunliffe NA, et al. Molecular analysis of the 18S rRNA gene of Cryptosporidium parasites from patients with or without human immunodeficiency virus infections living in Kenya, Malawi, Brazil, the United Kingdom, and Vietnam. J Clin Microbiol. 2003;41(4):1458–1462. doi: 10.1128/JCM.41.4.1458-1462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb DM, Steenhoff AP, Pernica JM, Chong S, Luinstra K, Mokomane M, et al. Evaluation of anatomically designed flocked rectal swabs for molecular detection of enteric pathogens in children admitted to hospital with severe gastroenteritis in Botswana. J Clin Microbiol. 2014;52:3922–3927. doi: 10.1128/JCM.01894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy YA, Krücken J, Nöckler K, von Samson-Himmelstjerna G, Zessin KH. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet Parasitol. 2013;193:15–24. doi: 10.1016/j.vetpar.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Karshima SN, Karshima MN. Epidemiology of Cryptosporidium infections among people living with HIV/AIDS in Nigeria: results of systematic review and meta-analysis. Acta Parasitol. 2020;66(4):60–74. doi: 10.1007/s11686-020-00253-8. [DOI] [PubMed] [Google Scholar]
- Kiros H, Nibret E, Munshea A, Kerisew B, Adal M. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at Felegehiwot Referral Hospital, Bahir Dar, Ethiopia. Int J Infect Dis. 2015;35:80–86. doi: 10.1016/j.ijid.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, et al. Burden and aetiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Leav BA, Mackay MR, Anyanwu A, O' Connor RM, Cevallos AM, Kindra G,, et al. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect Immun. 2002;70:3881–3890. doi: 10.1128/iai.70.7.3881-3890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikai BV, Umoh JU, Lawal IA, Kudi AC, Ejembi CL, Xiao L. Molecular characterizations of Cryptosporidium, Giardia, and Enterocytozoon in humans in Kaduna State. Nigeria Exp Parasitol. 2012;131(4):452–456. doi: 10.1016/j.exppara.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Masungo P, Dube T, Makaka C. A survey of the diversity of human enteric protoctistan parasites and the associated risk factors in urban Zvishavane. Zimbabwe Agric Biol J N Am. 2010;1(5):985–991. doi: 10.5251/abjna.2010.1.5.985.991. [DOI] [Google Scholar]
- Mele R, Morales MAG, Tosini F, Pozio E. Indinavir reduces Cryptosporidium parvum infection in both in vitro and in vivo models. Int J Parasitol. 2003;33(7):757–764. doi: 10.1016/s0020-7519(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Missaye A, Dagnew M, Alemu A, Alemu A. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending Dessie Hospital ART clinic, Northeast Ethiopia. AIDS Res Ther. 2013;10(1):7. doi: 10.1186/1742-6405-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–441. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Mokomane M, Kasvosve I, Gaseitsiwe S, Steenhoff AP, Pernica JM, Lechiile K, Luinstra K, Smieja M, Goldfarb DM. A comparison of flocked swabs and traditional swabs, using multiplex real time PCR for detection of common gastroenteritis pathogens in Botswana. Diagn Microbiol Infect Dis. 2016;86(2):142–143. doi: 10.1016/j.diagmicrobio.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan U, Weber R, Xiao L, Sulaiman I, Thompson RC, Ndiritu W, Lal A, Moore A, Deplazes P. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J Clin Microbiol. 2000;38:1180–1183. doi: 10.1128/jcm.38.3.1180-1183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse TD, Nichols RAB, Grimason AM, Campbell BM, Tembo KC, Smith HV. Incidence of cryptosporidiosis species in paediatric patients in Malawi. Epidemiol Infesict. 2007;135:1307–1315. doi: 10.1017/S0950268806007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msolo L, Iweriebor BC, Okoh AI. Rotavirus and Cryptosporidium pathogens as etiological proxies of gastroenteritis in some pastoral communities of the Amathole district municipality, Eastern Cape, South Africa. BMC Res Notes. 2020;13:187. doi: 10.1186/s13104-020-05024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtapuri-Zinyowera S, Ruhanya V, Midzi N, Berejena C, Chin'ombe N, Nziramasanga P, et al. Human parasitic protozoa in drinking water sources in rural Zimbabwe and their link to HIV infection. Germs. 2014;4:86–91. doi: 10.11599/germs.2014.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nchito M, Kelly P, Sianongo S, Luo NP, Feldman R, Farthing M, Baboo KS. Cryptosporidiosis in urban Zambian children: an analysis of risk factors. Am J Trop Med Hyg. 1998;59:435–437. doi: 10.4269/ajtmh.1998.59.435. [DOI] [PubMed] [Google Scholar]
- Odeniran PO, Ademola IO. Epidemiology of Cryptosporidium infection in different hosts in Nigeria: a meta-analysis. Parasitol Int. 2019;71:194–206. doi: 10.1016/j.parint.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Ojuromi TO, Ashafa AOT. Cryptosporidiosis in southern Africa: review of prevalence and molecular epidemiology of a neglected disease. Ann Trop Med Public Health. 2018;11(4):108–118. doi: 10.4103/atmph.atmph_29_17. [DOI] [Google Scholar]
- Peng MM, Meshnick SR, Cunliffe NA, Thindwa BD, Hart CA, Broadhead RL, et al. Molecular epidemiology of cryptosporidiosis in children in Malawi. J Eukaryot Microbiol. 2003;50(Suppl):557–559. doi: 10.1111/j.1550-7408.2003.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Samie A, Bessong PO, Obi CL, Sevilleja JE, Stroup S, Houpt E, et al. Cryptosporidium species: preliminary descriptions of the prevalence and genotype distribution among school children and hospital patients in the Venda region, Limpopo province, South Africa. Exp Parasitol. 2006;114(4):314–322. doi: 10.1016/j.exppara.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Samie A, Makuwa S, Mtshali S, Potgieter N, Thekisoe O, Mbati P, et al. Parasitic infection among HIV/AIDS patients at bela-bela clinic, Limpopo province, South Africa with special reference to Cryptosporidium. Southeast Asian J Trop Med Public Health. 2014;45:783–795. [PubMed] [Google Scholar]
- Samra NA, Jori F, Caccio SM, Frean J, Poonsamy B, Thompson PN. Cryptosporidium genotypes in children and calves living at the wildlife or livestock interface of the Kruger National Park. South Africa Onderste J Vet Res. 2016;83(1):a1024. doi: 10.4102/ojvr.v83i1.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samra NA, Thompson PN, Jori F, Frean J, Poonsamy B, du Plessis D, et al. Genetic characterization of Cryptosporidium spp. in diarrhoeic children from four provinces in South Africa. Zoonoses Public Health. 2013;60:154–159. doi: 10.1111/j.1863-2378.2012.01507.x. [DOI] [PubMed] [Google Scholar]
- Sinyangwe NN, Siwila J, Muma JB, Chola M, Michelo C. Factors associated with Cryptosporidium infection among adult HIV positive population in contact with livestock in Namwala district. Zambia Front Public Health. 2020;8:74. doi: 10.3389/fpubh.2020.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwila J, Phiri IG, Enemark HL, Nchito M, Olsen A. Seasonal prevalence and incidence of Cryptosporidium spp. and Giardia duodenalis and associated diarrhea in children attending pre-school in Kafue, Zambia. Trans R Soc Trop Med Hyg. 2011;105:102–108. doi: 10.1016/j.trstmh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Siwila J, Phiri IGK, Enemark HL, Nchito M, Olsen A. Intestinal helminths and protozoa in children in pre-schools in Kafue district, Zambia. Trans R Soc Trop Med Hyg. 2010;104:122–128. doi: 10.1016/j.trstmh.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Siwila J, Phiri IGK, Vercruysse J, Goma F, Gabriel S, Claerebout E, Geurden T. Asymptomatic cryptosporidiosis in Zambian dairy farm workers and their household members. Trans R Soc Trop Med Hyg. 2007;101:733–734. doi: 10.1016/j.trstmh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, et al. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS) PLoS Negl Trop Dis. 2016;10(5):e0004729. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire SA, Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vect. 2017;10:195. doi: 10.1186/s13071-017-2111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CR, Lechiile K, Mokomane M, Steenhoff AP, Arscott-Mills T, Pernica JM, Goldfarb DM. Evaluation of anatomically designed flocked rectal swabs for use with the biofire filmarray gastrointestinal panel for detection of enteric pathogens in children admitted to hospital with severe gastroenteritis. J Clin Microbiol. 2019;57:e00962–e1019. doi: 10.1128/JCM.00962-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2015) World Health Organisation, Geneva Switzerland. World Health Statistics. http://apps.who.int/iris/bitstream/10665/170250/1/9789240694439_eng.pdf. Accessed 25 Sept 2016.
- Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.