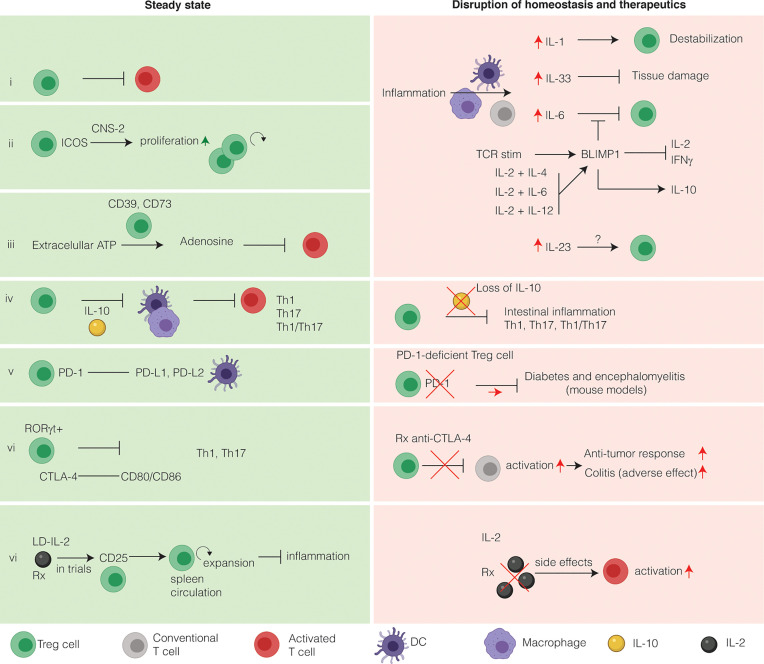
Figure 2.
Intestinal Treg cells tightly maintain intestinal immune homeostasis and relevance for immunotherapy. Intestinal Treg cells have several markers that discriminate them from other Treg cells. Under homeostatic conditions, Treg cells suppress effector T cells to prevent inflammation (i). During inflammation, several inflammatory cytokines alter the phenotype of intestinal Treg cells. (ii) Inducible co-stimulator (ICOS) stabilizes intestinal Treg cells in a CNS2-dependent manner. (iii) Extracellular ATP is converted to the immunosuppressive adenosine by Treg cells expressing CD39 and CD73. CD73 is induced by TGFβ. (iv) The main immunosuppressive cytokine secreted by intestinal Treg cells is IL-10. IL-10 functions to inhibit T-helper 1 (Th1), Th17 and Th1/Th17 inflammation in a STAT3-dependent manner via IL-10R signaling on APCs, limiting inflammasome activation. On the other hand, loss of IL-10 both in mice and in humans leads to intestinal inflammation and IBD, respectively. The main co-stimulatory receptors expressed on Treg cells are programmed death receptor (PD-1) (v) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) (vi). Both these co-stimulatory receptors are exploited therapeutically to increase the anti-cancer immune response. Anti-CTLA-4 therapy has colitis as a frequent side-effect, whereas colitis is not associated with blockade of the PD-1 pathway. (vi) Because Treg cells express the high affinity receptor CD25, they respond to low doses of IL-2 by expansion, thus limiting inflammation. LD-IL-2 is investigated in a clinical trial for its use in patients with IBD. In contract to LD-IL-2, a high dose of IL-2 will activate T cells and lead to deleterious side effects and therefore is not used in clinical practice.