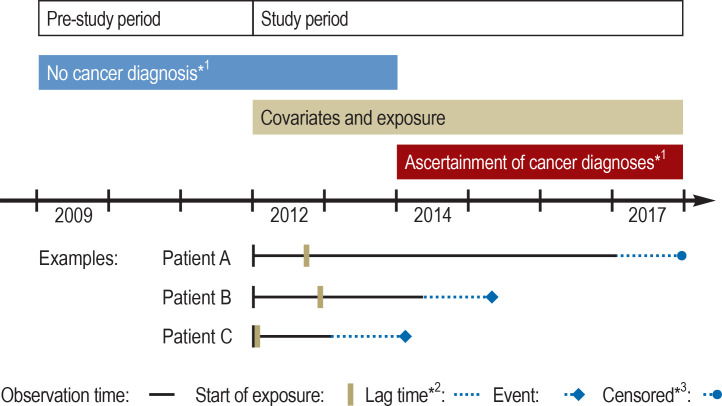
Figure.
Overview of the study design
*1 For the evaluation of long-term use (3 years) the absence of cancer until the end of 2014 was required; follow-up started at the beginning of 2015.
*2 Depicted is the lag time of the main analysis (lag time of 1 year); for the evaluation of long-term use (3 years), no lag time was included.
*3 Data censored because of death, end of insurance cover, or end of study.