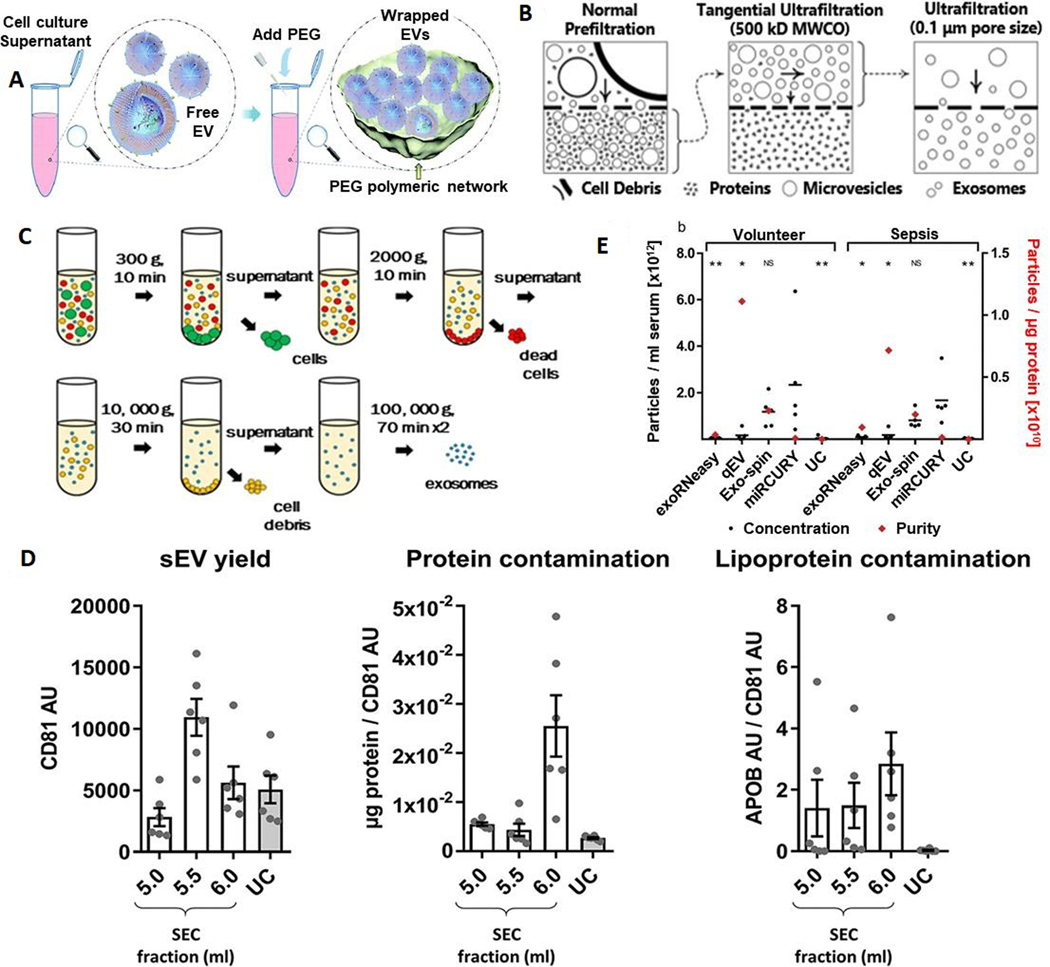
Figure 3.
Conventional methods for EV enrichment. (A) Polymer-based enrichment: Precipitation with polyethylene glycol (PEG) (reproduced from Reference 100). (B) Filtration and ultrafiltration for EV isolation: normal prefiltration can collect sEVs and particles into the bottom layer of the culture dish. The bottom layer needs to be processed through tangential ultrafiltration, and the retentate is collected. Further ultrafiltration with expected pore size can be further processed and the EVs with a size smaller than the pore size will be present in the permeate (reproduced from[28]). (C) Ultracentrifugation for EV isolation (Reproduced from[111]). (D) Summary of yield and purity of sEVs isolated by SEC or UC: Normalization of APOB signal to CD81 content as an estimate of sEV purity from lipoproteins, also demonstrated almost 60 times higher APOB/CD81 ratio in the peak sEV fraction of SEC (5.5 ml) compared to the UC samples. SEC resulted in a higher yield of sEVs but with marked contamination by soluble protein and lipoproteins (reproduced from[116]). (E) Analysis of EVs by NTA demonstrates differences in size distribution. Black bars indicate the absolute number of vesicles isolated from 1 ml of serum; red diamonds plotted against the right x-axis represent vesicle purity defined as the particle to protein ratio. While precipitation most efficiently isolated EVs from serum, SEC-based isolation yielded fewer but more pure vesicles. Asterisks indicate significant differences in particle numbers compared to miRCURY. *P < 0.05; **P < 0.01; NS: not significant. All data are mean ±SD for five volunteers and five sepsis patients (reproduced from[117]). NTA: Nanoparticle tracking analysis.